The Use of Platelet-Rich Plasma Alone in The Treatment of Periodontal Endosseous Defects: A Report of Two Clinical Cases
A B S T R A C T
Background: On the basis of animal histologic data and randomized controlled trials, the authors provide reports of two cases, in which platelet-rich plasma (PRP) alone was used to treat periodontal endosseous defects in patients exhibiting generalized severe chronic periodontitis.
Case Descriptions: Clinicians treated endosseous defects with a probing pocket depth of 6 and 8 millimeters (case 1), and 4 mm (case 2) by using PRP alone. At six months, complete periodontal pocket resolution, and significant clinical attachment gain and radiographic defect fill, compared with pre-surgical values, occurred in all defects.
Clinical Implications: PRP alone may be clinically and radiographically efficacious in the treatment of periodontal endosseous defects.
Keywords
Periodontitis, periodontal treatment, monotherapy, periodontal endosseous defects, platelet-rich plasma
Introduction
Platelet-rich plasma (PRP) is a medical and dental preparation produced by a two-step centrifugation of the blood of the patient and used as an autologous source of platelets in high concentrations [1-4]. A first centrifugation separates the underlying erythrocytes from the PRP layer in the middle and the platelet-poor plasma (PPP) layer at the top [5]. A second centrifugation separates the underlying PRP from the overlying PPP [5]. Following centrifugations, the concentration of platelets in PRP has been reported to increase to a level ranging from 275% to 360%, relative to their concentration in the patient’s blood before any centrifugation [4, 6, 7]. Coagulation activators, usually calcium chloride (CaCl2) mixed with thrombin or, if surgery is being performed concomitantly, the patient’s own blood harvested from the surgical site, activate the platelets contained in PRP [4]. Subsequently, the activated platelets release various polypeptide growth factors, presynthesized and stored within their α granules [3]. The main growth factors of PRP are platelet-derived growth factor, transforming growth factors -β1 and -β2 and insulin-like growth factor-1) [2-4].
As a result of the biological activity of these growth factors, as well as other mechanisms not completely elucidated yet, PRP has the potential to enhance and accelerate healing processes of both soft tissues (such as epithelialization) and hard tissues (such as osseous regeneration) [2, 4, 8-11]. Therefore, PRP often limits inflammation and provides an improved aesthetic outcome and a shortened duration of therapy [8, 9, 12]. To date, two systematic reviews and a conventional (not systematic) review have examined the adjunctive use of PRP (i.e. the addition of PRP to various bioactive agents) in the therapy of periodontal endosseous defects in chronic periodontitis patients [4, 13, 14]. These reviews demonstrated that both the presence and the absence of statistically significant adjunctive benefits had been reported by original research studies, when the efficacy of PRP in combination with bioactive agent(s) was compared with the efficacy of the same bioactive agent(s), without the addition of PRP [4, 13, 14]. This variance in study outcomes may possibly originate from the limited amount of data existing at present or could also imply that the type/nature of bioactive agents combined with PRP could be important [4].
Along with the adjunctive use of PRP in the therapy of periodontal endosseous defects, the exclusive use of PRP in treating such defects should also be given due consideration. Most recently, the first histologic data were published, indicating the potential of platelet pellet (which contains platelets in concentrations approximately 15- to 30-fold higher than in PRP and, furthermore, is more adhesive than PRP), when used as a monotherapy for chronic Class II furcation defects in dogs, to induce the formation of all supporting periodontal tissues (new cementum of variable thickness, new connective tissue attachment with intrinsic and extrinsic collagen fibers randomly inserting into the cementum and, finally, limited amounts of alveolar bone forming coronally), without root resorption or ankylosis [15]. In line with these histologic data, in recent randomized controlled trials (RCTs), endosseous defects or mandibular degree II furcation defects were treated with PRP alone and, post-operatively, both clinical (mean probing pocket depth (PPD) reduction and mean clinical attachment level (CAL) improvement) and radiographic (mean defect fill) parameters of efficacy exhibited statistically and clinically significant mean improvement, compared with baseline values [16-20]. In light of the outcomes of these studies, it appears that PRP has the potential of being used as a monotherapy of periodontal endosseous defects [16-20].
We previously reported on two clinical cases, in which the combination of PRP and DFDBA was used for the treatment of periodontal endosseous defects in systemically healthy non-smoking patients with generalized severe chronic periodontitis [21]. In this article, we report on two clinical cases, in which PRP alone was used to treat periodontal endosseous defects in systemically healthy non-smoking patients with generalized severe chronic periodontitis.
Report of clinical cases
All procedures performed in patients in cases 1 and 2, as described below, had been approved by the Ethics and Research Committee, School of Dentistry, University of Athens, Greece, as mentioned in a previous study [19]. Patients provided written informed consent. We selected both the patients and the periodontal endosseous defects in cases 1 and 2 in such a way as to present characteristics in line with our previously used inclusion criteria [19]. For both clinical cases reported in this article, a single laboratory operator (E.P.) carried out the preparation of PRP by using a standardized kit (PRP kit, Curasan, Kleinostheim, Germany) and applying methods also previously described [19, 21].
Clinical Cases
Table 1 presents patient data for both clinical cases, including full-mouth plaque scores (as proposed by O’Leary and colleagues) and gingival scores (as described by Ainamo and Bay) [22, 23]. Tables 2 and 3 present defect data (baseline and final) for clinical case 1 and clinical case 2, respectively. Figures 1 through 6 are the clinical views for clinical case 1, and (Figures 7 to 9) are the radiographic images for the same clinical case. Figures 10 through 14 are the clinical views for clinical case 2, and (Figures 15 to 17) are the radiographic images for the same clinical case.
I Clinical Case 1
In the first clinical case, a male patient, aged 54 years, sought periodontal therapy in the Department of Periodontology, School of Dentistry, University of Athens, Greece. The patient was systemically healthy and was not receiving treatment with antibiotics or other medications. Furthermore, the patient was not a smoker and had generalized severe chronic periodontitis, according to established criteria [24].
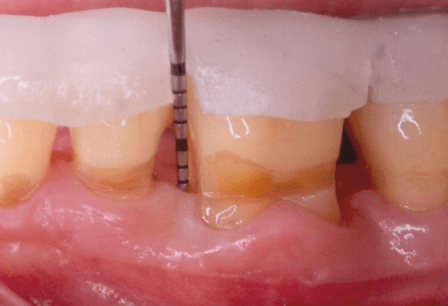
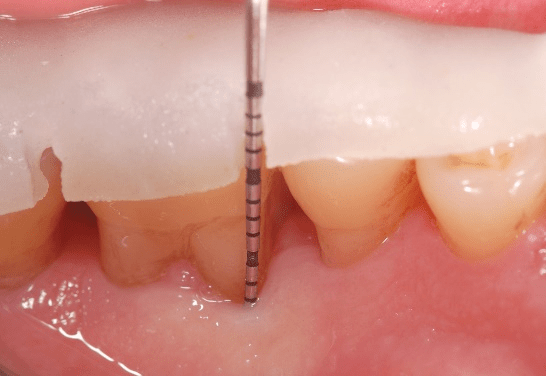
Figure 1: Case 1: baseline (pre-surgical) facial clinical view of the periodontal endosseous defects, located mesial and distal to tooth no. 19. Probing pocket depth measurement of 6 millimeters mesio-facial to the tooth and of 8 millimeters disto-facial to the tooth.
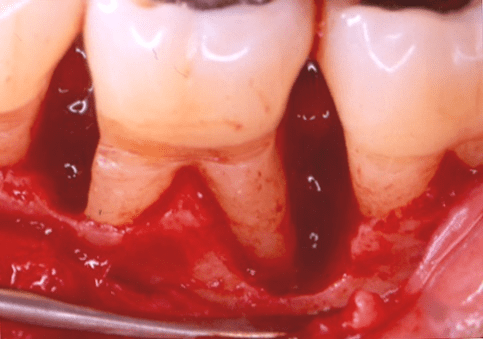
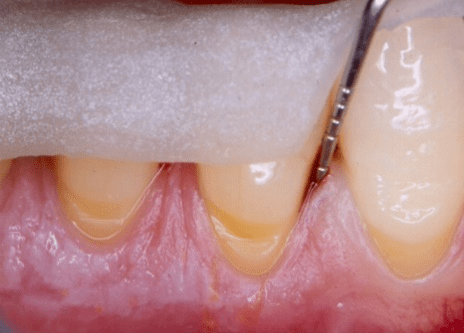
Figure 2: Facial clinical view of the two-wall endosseous defects located mesial and distal to tooth no. 19 in the patient in case 1, after the elevation of a full-thickness flap with concomitant papilla preservation and meticulous debridement and root planing, and immediately before the defects were filled with platelet-rich plasma alone.
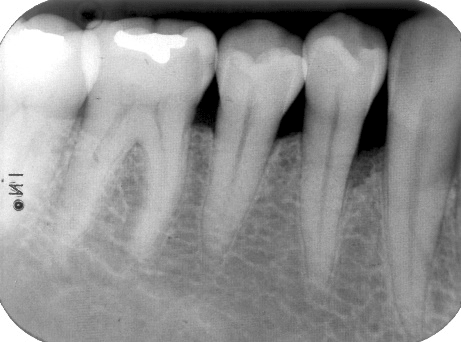
The periodontal endosseous defects selected to be treated with PRP alone were located mesial and distal to tooth no. 19 (Figures 1 and 2). A single calibrated clinical examiner (E.P.) performed all clinical measurements. The examiner recorded baseline defect data (Table 2), depending on the parameter determined, either pre-surgically (i.e. at six weeks after scaling and root planing) (Figure 1) or intra-surgically (i.e. just before the operator filled each defect with PRP alone) (Figure 2). In brief, both endosseous defects had two osseous walls (Figure 2) (Table 2), exhibited advanced (6 mm or greater) CAL and PPD measurements (Table 2) and advanced (3 mm or greater) radiographic depth of their endosseous component (Figure 7). The clinical examiner performed these clinical measurements at six sites per tooth (mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual and disto-lingual) and rounded them off to the nearest millimeter [19]. An occlusal acrylic stent was prepared for each defect and had two guiding grooves at specific sites (facially and lingually) [19]. The most apical border of each groove served as a fixed reference point for the above-mentioned clinical measurements (Figure 1) [19]. Furthermore, tooth no. 19 had degree 1 mobility (movability of the dental crown of 0.2 to 1 millimeter in a horizontal direction, according to the classification of tooth mobility grades proposed by Nyman and colleagues) without occlusal interferences [25]. The above-mentioned radiographic determinations were made by a single calibrated operator (H.C.S.) using a baseline periapical radiograph (Figure 7), obtained under standardized conditions [19].
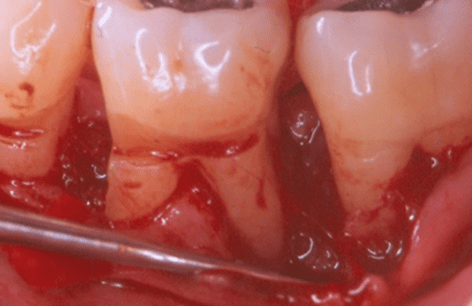
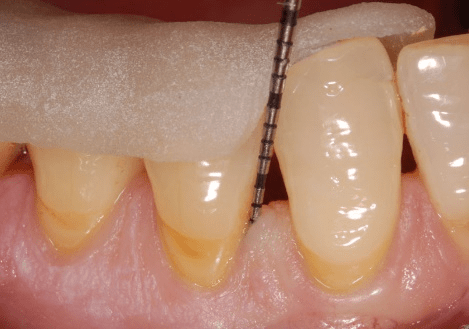
Figure 3: Facial clinical view of the endosseous defects mesial and distal to tooth no. 19 in the patient in case 1, completely filled with platelet-rich plasma alone.
A single clinical operator (N.M.) performed the surgical procedure. The surgery included intra-sulcular incisions, full-thickness flap elevation with concomitant papilla preservation, meticulous debridement and root planing (Figure 2), endosseous defect filling with PRP alone (Figure 3), flap repositioning and suturing by means of a modified vertical mattress and an interrupted suturing technique [19]. During the post-surgical period, the clinical operator systemically administered doxycycline hyclate for 10 days (100 milligrams every 12 hours on the day of the surgery and every 24 hours throughout the subsequent nine days) [19]. Post-operative healing was uneventful for both endosseous defects. The clinical examiner re-examined the patient every two weeks throughout the first month and once per month thereafter, up to and including six months post-operatively; no adverse events or complications occurred during this follow-up period [19].
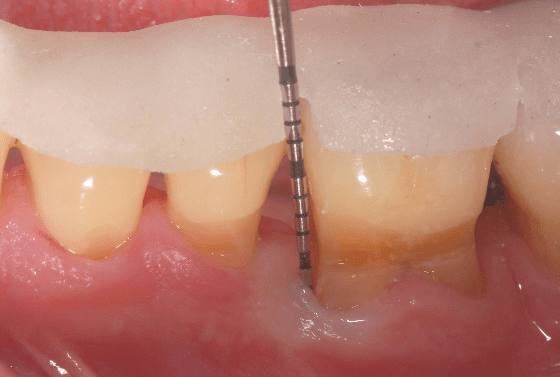
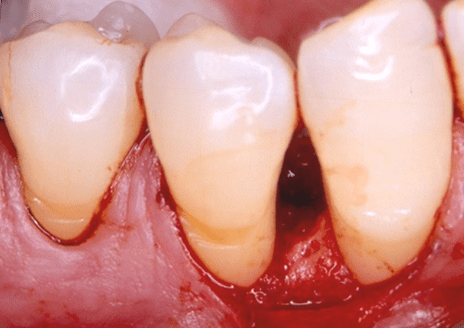
Figure 4: Final (six-month post-surgical) facial clinical view of tooth no. 19 in the patient in case 1 depicting complete periodontal pocket resolution mesial to the tooth (probing pocket depth of 3 millimeters or less).
Figure 5: Final (six-month post-surgical) lingual clinical view of tooth no. 19 in the patient in case 1 depicting complete periodontal pocket resolution mesio-lingual to the tooth (probing pocket depth of 3 millimeters or less).
Figure 6: Final (six-month post-surgical) lingual clinical view of tooth no. 19 in the patient in case 1 depicting complete periodontal pocket resolution disto-lingual to the tooth (probing pocket depth of 3 millimeters or less).
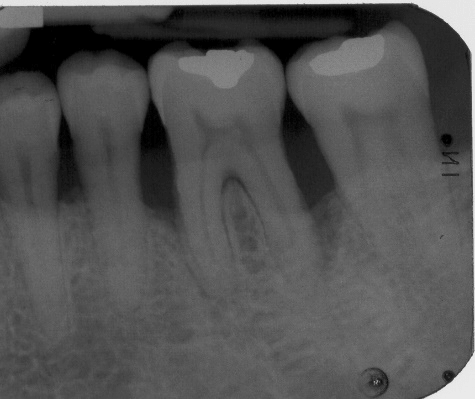
Figure 7: Baseline (pre-surgical) periapical radiograph of periodontal endosseous defects, located mesial and distal to tooth no. 19 in the patient in case 1.
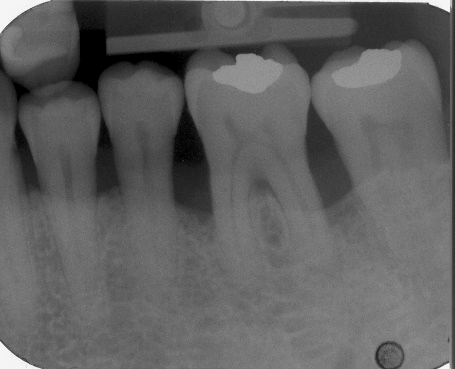
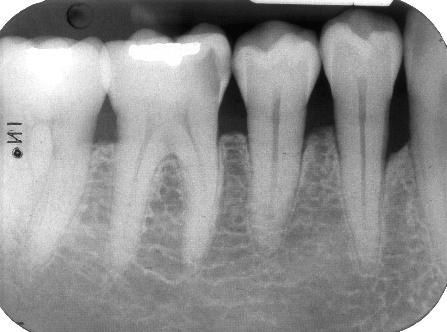
Figure 8: Final (six-month post-surgical) periapical radiograph of periodontal endosseous defects, located mesial and distal to tooth no. 19.
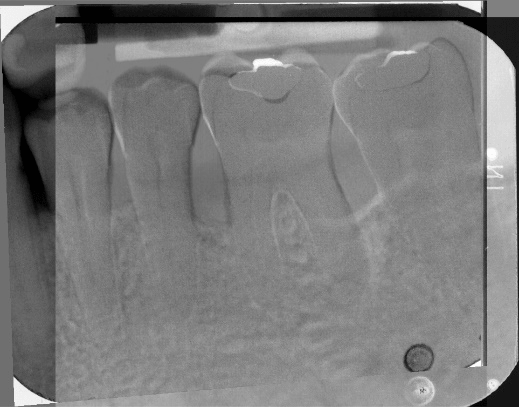
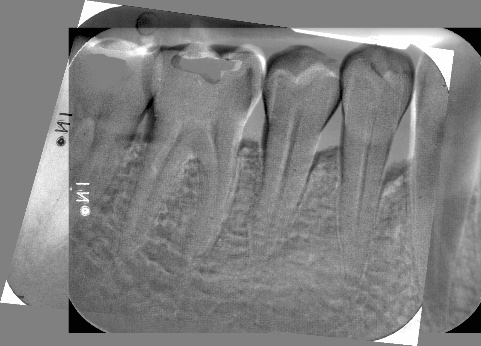
Figure 9: Subtraction radiograph illustrating the difference between Figures 7 and 8, indicates defect fill of periodontal endosseous defects located mesial and distal to tooth no. 19 in the patient in case 1.
The aforementioned clinical examiner (E.P.) recorded final (six-month) patient data (Table 1) and defect data (Table 2) (Figures 4 to 6), except for data derived from the final periapical radiograph (Figure 8). A single operator (H.C.S.) obtained all radiographs and performed all radiographic measurements, analyses and other procedures. This operator used subtraction radiography (Figure 9) to highlight the differences between the baseline radiograph (Figure 7) and the final periapical radiograph (Figure 8) by applying previously described methods [19]. In brief, the operator transformed the baseline and final periapical radiographs into the baseline digitized image and the final digitized image, respectively, both to a resolution of 300 dots per inch with eight bits of gray-level resolution per pixel [19]. The baseline digitized image then served as the reference image, whereas the operator reconstructed the final digitized image for each endosseous defect, according to its reference image, by using a software program (Emago, version 3.1, Oral Diagnostic Systems, Amsterdam, The Netherlands) that provided geometric standardization [19].
Table 1: Patient data for clinical cases 1 and 2.
|
CHARACTERISTIC |
CASE 1 |
CASE 2 |
|
Age (Years) |
54 |
55 |
|
Sex |
Male |
Male |
|
Smoking Status (Never, Former or Active Smoker; Daily Cigarette Consumption (Number); Smoking Duration (Years))* |
Never 0 0 |
Active 10 10 |
|
Full-Mouth Plaque Score (Percentage of Total Tooth Surfaces With Plaque)† Before scaling and root planing (SRP) Before surgery Final |
10% 8% 5% |
82%; 16%; 14% |
|
Gingival Score (Percentage of Total Tooth Surfaces With Bleeding)‡ Before SRP Before surgery Final |
11% 9% 6% |
75% 15% 12% |
|
Full-mouth Bleeding on Probing Score (Percentage of Total Tooth Surfaces With Bleeding on Probing)§ Before SRP Before surgery Final |
13% 10% 8% |
78% 19% 18% |
Abbreviations:
GI: Gingival index, according to Ainamo & Bay [22].
* Smoking data were recorded at the initial patient examination (before the patients underwent any therapy). For the patient in clinical case 1, smoking data remained unaltered through the final examination, whereas for the patient in clinical case 2, the final daily cigarette consumption was 10 cigarettes per day, i.e. was slighly reduced, compared with the initial value.
† Full-mouth plaque score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of plaque, when the clinician ran a periodontal probe tip on the tooth surface along the gingival margin. This score was a slight modification of the original “plaque control record” proposed by O’Leary and collegues, in which four aspects per tooth had been examined [22].
‡ Gingival score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of bleeding, when the clinician ran a periodontal probe tip on the tooth surface along the gingival margin (recorded according to the method of Ainamo and Bay) [23].
§ Full-mouth bleeding on probing score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of bleeding within 30 seconds after the clinician probed at the base (the most apical border) of each periodontal pocket.
At six months, in both endosseous defects complete periodontal pocket resolution (PPD of 3 millimeters or less) occurred (Table 2) (Figures 4 to 6) and, furthermore, CAL (Table 2) (Figures 4 to 6) and radiographic defect fill (Figures 8 and 9) exhibited significant improvement, compared with the corresponding pre-surgical values (Table 2) (Figures 1 and 7).
II Clinical Case 2
As noted previously, (Tables 1 and 3) present data for clinical case 2, and (Figures 10 to 17) are images related to this case. In clinical case 2, a male patient, aged 55 years, sought periodontal therapy in the Department of Periodontology, School of Dentistry, University of Athens, Greece. The patient was systemically healthy and was not receiving treatment with antibiotics or other medications. Furthermore, the patient was smoking lightly (smoked 10 cigarettes per day or less) and presented generalized severe chronic periodontitis, according to established criteria [24].
Table 2: Defect data (baseline and final) for clinical case 1 (tooth no. 19).
|
TREATED TOOTH (ASPECT) / PARAMETER |
BASELINE DATA (Pre-surgical & Intra-surgical) |
FINAL DATA (POST-SURGICAL) |
|
Tooth no. 19 (Mesial) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
Clinical attachment level* (millimeters) |
10, 5, 13, 11, 5, 10 |
7, 5, 7, 8, 5, 7 |
|
Probing pocket depth* (mm) |
6, 2, 8, 6, 2, 6 |
3, 2, 3, 3, 2, 3 |
|
Gingival recession depth (apicocoronal)* (mm) |
4, 3, 5, 5, 3, 4 |
4, 3, 4, 5, 3, 4 |
|
Bleeding on probing* (present, +; absent, -) |
+, -, +, +, +, + |
-, -, -, -, -, + |
|
Furcation involvement† (no furcation defect, 0; furcation defect class, I, II or III) |
0, 0 |
0, 0 |
|
Mobility (no mobility, 0; mobility degree, 1, 2 or 3) |
1 |
1 |
|
Keratinized tissue width* (mm) |
3, 5, 4, 5, 4, 4 |
3, 5, 4, 5, 4, 4 |
|
Keratinized tissue thickness* (≥1 mm, +; <1 mm, -) |
+, +, +, +, +, + |
+, +, +, +, +, + |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
2 (facial and mesial) |
|
|
Tooth surfaces (number, location) |
1 (mesial) |
|
|
Distance from the acrylic stent to the alveolar crest‡ (mm) |
10, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base‡ (mm) |
15, 15 |
|
|
Distance from the alveolar crest to the endosseous defect base‡ (mm) |
5, 5 |
|
|
Width of the endosseous defect§ (mm) Faciopalatal Mesiodistal† |
9 5, 4 |
|
|
|
|
|
|
Tooth no. 19 (Distal) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
|
All pre-surgical data for the endosseous defect located distal to tooth no. 19 coincide with the pre-surgical data reported above for the endosseous defect located mesial to the same tooth |
All post-surgical data for the endosseous defect located distal to tooth no. 19 coincide with the post-surgical data reported above for the endosseous defect located mesial to the same tooth |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
2 (facial and distal) |
|
|
Tooth surfaces (number, location) |
3 (facial, distal and lingual) |
|
|
Distance from the acrylic stent to the alveolar crest¶ (mm) |
14, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base¶ (mm) |
18, 17 |
|
|
Distance from the alveolar crest to the endosseous defect base¶ (mm) |
4, 7 |
|
|
Width of the endosseous defect§ (mm) Faciopalatal Mesiodistal† |
9 5, 5 |
|
Abbreviations:
* Measurements at six sites per tooth, reported according to the sequence: mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual and disto-lingual.
† Measurements on two tooth aspects, reported according to the sequence: facial and lingual.
‡ Measurements at two sites per tooth (only mesial, because the endosseous defect was located mesially), reported according to the sequence: mesio-facial and mesio-lingual.
§ Measured at the level of the alveolar crest.
¶ Measurements at two sites per tooth (only distal, because the endosseous defect was located distally), reported according to the sequence: disto-facial and disto-lingual.
Table 3: Defect data (baseline and final) for clinical case 2 (tooth no. 28).
|
TREATED TOOTH (ASPECT) / PARAMETER |
BASELINE DATA (Pre-surgical & Intra-surgical) |
FINAL DATA (POST-SURGICAL) |
|
Tooth no. 28 (Mesial) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
Clinical attachment level* (millimeters) |
6, 4, 3, 5, 5, 3 |
6, 4, 3, 4, 3, 2 |
|
Probing pocket depth* (mm) |
4, 1, 3, 3, 3, 3 |
2, 1, 2, 0, 1, 1 |
|
Gingival recession depth (apicocoronal)* (mm) |
2, 3, 0, 2, 2, 0 |
4, 3, 1, 4, 2, 1 |
|
Bleeding on probing* (present, +; absent, -) |
+, -, -, +, -, - |
-, -, -, -, -, - |
|
Mobility (no mobility, 0; mobility degree, 1, 2 or 3) |
0 |
0 |
|
Keratinized tissue width* (mm) |
7, 2, 8, 7, 4, 8 |
7, 2, 8, 7, 4, 8 |
|
Keratinized tissue thickness* (≥1 mm, +; <1 mm, -) |
+, +, +, +, +, + |
+, +, +, +, +, + |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
Combination 1-2, mesial – (mesial and facial) |
|
|
Tooth surfaces (number, location) |
1 (mesial) |
|
|
Distance from the acrylic stent to the alveolar crest† (mm) |
10, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base† (mm) |
13, 14 |
|
|
Distance from the alveolar crest to the endosseous defect base† (mm) |
3, 4 |
|
|
Width of the endosseous defect‡ (mm) Faciopalatal Mesiodistal§ |
5 3, 3 |
|
Abbreviations:
* Measurements at six sites, reported according to the sequence: mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual and disto-lingual.
† Measurements at two sites (only mesial, because the endosseous defect was located mesially), reported according to the sequence: mesio-facial and mesio-lingual.
‡ Measured at the level of the alveolar crest.
§ Measurements on two tooth aspects, reported according to the sequence: facial and lingual.
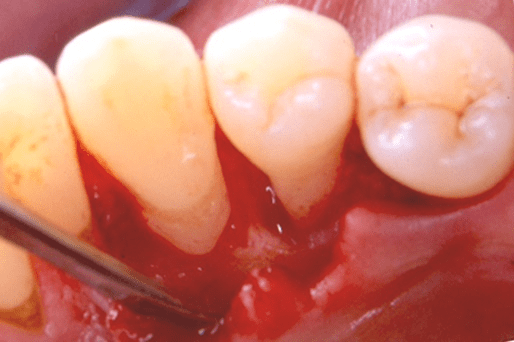
The periodontal endosseous defect selected to be treated with PRP alone was located mesial to tooth no. 28 (Figure 10). Table 3 provides detailed baseline defect data (pre-surgical and intra-surgical). The endosseous defect presented a combination of one-to-two osseous walls (Figures 11 and 12), moderate CAL (5-6 millimeters) and shallow PPD (3-4 millimeters) measurements (Table 3; Figure 10) and advanced (3 mm or greater) radiographic depth of its endosseous component (Figure 15). A single clinical operator (N.M.) performed the surgical procedure. The surgery included intra-sulcular incisions, full-thickness flap elevation with concomitant papilla preservation, meticulous debridement and root planing (Figures 11 and 12), endosseous defect filling with PRP alone (Figure 13), flap repositioning and suturing by means of a modified vertical mattress and an interrupted suturing technique [19].
During the post-surgical period, the clinical operator (N.M.) systemically administered doxycycline hyclate for 10 days, using the regimen described above for the patient in clinical case 1 [19]. Post-operative healing was uneventful. The clinical examiner (E.P.) re-examined the patient at the same frequency as that in clinical case 1; no adverse events or complications occurred during the six-month follow-up period. The clinical examiner recorded the final (six-month) patient data (Table 1) and defect data (Table 3, Figure 14), except for data derived from the final periapical radiograph (Figure 16). A single operator (H.C.S.) obtained all radiographs and performed all radiographic measurements, analyses and other procedures. This operator used subtraction radiography (Figure 17) to highlight the differences between the baseline periapical radiograph (Figure 15) and the final periapical radiograph (Figure 16) [19].
Figure 10: Case 2: baseline (pre-surgical) facial clinical view of the periodontal endosseous defect located mesial to tooth no. 28. Probing pocket depth measurement of 4 millimeters mesio-facial to the tooth.
Figure 11: Facial clinical view of the endosseous defect located mesial to tooth no. 28 in the patient in case 2 after the elevation of a full-thickness flap with concomitant papilla preservation, revealing the presence of two osseous walls (mesial and facial).
Figure 12: Lingual clinical view of the endosseous defect located mesial to tooth no. 28 in the patient in case 2 after the elevation of a full-thickness flap with concomitant papilla preservation, revealing the presence of one osseous wall (mesial). Collectively, Figures 11 and 12 reveal that this endosseous defect presents a combination of one-to-two osseous walls.
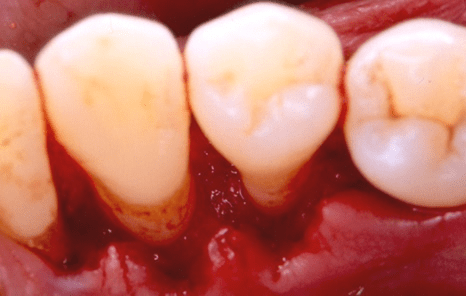
At six months, in the endosseous defect complete periodontal pocket resolution occurred (Table 3, Figure 14) and CAL exhibited significant improvement (Table 3, Figure 14), compared with the corresponding pre-surgical values (Table 3, Figure 10). Furthermore, as revealed by subtraction radiography (Figure 17), the alveolar crest mesial to tooth no. 28 was more radiopaque at six months (Figure 16), compared with the pre-surgical radiographic image (Figure 15), possibly suggesting osseous regeneration.
Figure 13: Lingual clinical view of the endosseous defect mesial to tooth no. 28 in the patient in case 2 completely filled with platelet-rich plasma alone.
Figure 14: Final (six-month post-surgical) facial clinical view of tooth no. 28 in the patient in case 2, depicting complete periodontal pocket resolution (probing pocket depth of 3 millimeters or less).
Figure 15: Baseline (pre-surgical) periapical radiograph of the periodontal endosseous defect located mesial to tooth no. 28 in the patient in case 2.
Figure 16: Final (six-month post-surgical) periapical radiograph of the periodontal endosseous defect located mesial to tooth no. 28 in the patient in case 2.
Figure 17: Subtraction radiograph revealing that the alveolar crest mesial to tooth no. 28 was more radiopaque at six months after surgery (Figure 16), compared with the pre-surgical radiographic image (Figure 15), possibly suggesting osseous regeneration.
Discussion
In this article, we report on two clinical cases illustrating the use of PRP as a monotherapy of periodontal endosseous defects. At six months, in both cases complete periodontal pocket resolution occurred, and CAL exhibited clinically significant improvement, compared with pre-surgical values. Furthermore, radiographic improvement was revealed for all defects by means of subtraction radiography. The safety of the use of PRP in the treatment of periodontal endosseous defects has been already addressed in a previous case report [21]. Therefore, this discussion will focus mainly on the efficacy of PRP alone in the treatment of periodontal endosseous defects.
The obtained outcomes in clinical cases 1 and 2 are in agreement with the findings of previous studies [16-20]. In particular, in a split-mouth randomized prospective case series (as characterized by the authors themselves; however, it might also be regarded as a split-mouth RCT, based on proposed definitions of study designs), 5 endosseous defects in 5 patients were treated with PRP alone and at 52 weeks post-operatively efficacy parameters exhibited significant mean improvement, compared with baseline values, both clinically (mean PPD reduction: 3.0 mm ± 1.41 mm and mean CAL improvement: 2.20 mm ± 1.79 mm) and radiographically (mean defect fill: 3.24 mm ± 2.85 mm) [16, 26]. In a parallel-group RCT, 12 endosseous defects in 8 patients were treated with PRP alone and at 18 months post-operatively, efficacy parameters exhibited significant mean improvement, compared with baseline values, both clinically (mean PPD reduction: 2.1 mm ± 0.5 mm and mean CAL improvement: 1.5 mm ± 0.7 mm) and radiographically (mean defect fill: 0.8 mm ± 0.8 mm) [17]. In a split-mouth RCT, 14 endosseous defects in 14 patients were treated with PRP alone and at 9 months post-operatively efficacy parameters exhibited significant mean improvement, compared with baseline values, both clinically (mean PPD reduction: 3.50 mm ± 0.65 mm and mean CAL improvement: 3.21 mm ± 0.58 mm) and radiographically, using spiral computed tomography (mean defect fill: 1.96 mm ± 0.26 mm and percent mean defect fill: 43.81% ± 8.85%; mean defect resolution: 1.97 mm ± 0.15 mm and percent mean defect resolution: 44.37% ± 8.39%) [18]. In another split-mouth RCT of the same research group, 20 mandibular degree II furcation defects in 20 patients were treated with PRP alone and at 6 months post-operatively efficacy parameters exhibited significant mean improvement, compared with baseline values, both clinically (mean vertical PPD reduction: 2.30 mm ± 1.41 mm; mean vertical CAL improvement: 2.50 mm ± 1.64 mm and mean horizontal CAL improvement: 2.50 mm ± 1.17 mm) and radiographically, using spiral computed tomography (mean vertical defect fill: 1.23 mm ± 0.43 mm and percent mean vertical defect fill: 32.08% ± 10.37%; mean horizontal defect fill: 1.33 mm ± 0.93 mm and percent mean horizontal defect fill: 28.74% ± 21.09%) [20]. Finally, in a most recent parallel-group RCT, 12 endosseous defects in 12 patients were treated with PRP alone and at 6 months post-operatively, efficacy parameters exhibited significant mean improvement, compared with baseline values, both clinically (mean PPD reduction: 3.92 mm ± 1.10 mm and mean CAL improvement: 3.08 mm ± 0.95 mm) and radiographically (percent mean defect fill: 41.29% ± 17.33%) [19].
When viewed as a collective body of evidence, these RCTs demonstrated that at time-points ranging from 6 to 18 months after the treatment of endosseous defects with PRP alone, efficacy parameters exhibited significant mean improvement, compared with the corresponding baseline values, both clinically (mean PPD reduction ranging from 2.10 mm up to 3.92 mm and mean CAL improvement ranging from 1.50 mm up to 3.21 mm) and radiographically (mean defect fill ranging from 0.80 mm up to 3.24 mm) [16-20]. The direct comparison of the efficacy of PRP alone to the efficacy of open flap debridement (which is generally regarded as the “gold standard” for the evaluation of the surgical treatment outcome in periodontal endosseous defects) has been performed in mandibular Class II furcation defects, demonstrating statistically significantly higher clinical and radiographic efficacy of PRP [20]. However, such a direct comparison has never been performed in periodontal endosseous defects and specially designed RCTs are certainly required on this issue. Despite this deficiency in the available evidence, an indirect comparison between the two treatment modalities is still feasible, if results from a previous meta-analysis are used as a historical control group [27]. According to this meta-analysis, open flap debridement alone resulted in limited (non-significant) PPD reduction, CAL improvement averaging 1.5 mm and osseous defect fill averaging 1.1 mm [27]. These mean values are approximately two to three times lower than the corresponding values mentioned above for PRP alone, thereby suggesting that the clinical and radiographic efficacy of PRP as a monotherapy of periodontal endosseous defects is not only statistically significant but could also be clinically significant.
Furthermore, it has to be pointed out that improvements in parameters of efficacy noted in individual clinical cases can be much higher than the mean values reported in research, as actually was confirmed in the clinical cases reported in the present article [28]. The estimation of the clinical significance of the outcomes obtained with PRP alone can be made by reporting the percentage of individual periodontal endosseous defects, demonstrating CAL or PPD improvements ≥2 mm or ≥3 mm [19]. Hence, all endosseous defects (100%) treated with PRP alone demonstrated CAL improvement ≥2 mm and 66.66% of the endosseous defects treated with PRP alone demonstrated CAL improvement ≥3 mm [19]. These results underscored the clinical significance of PRP as a monotherapy for periodontal endosseous defects.
In support of the favorable clinical and radiographic outcomes, most recent histologic data have revealed the potential of platelet pellet to promote regeneration of all supporting periodontal tissues (new cementum, new connective tissue attachment with intrinsic and extrinsic collagen fibers randomly inserting into the cementum and, finally, limited amounts of alveolar bone forming coronally) in Class II furcation defects in dogs, without root resorption or ankylosis [15]. The regenerative potential of platelet pellet was attributed to its sticky consistency, favoring its adhesion to the root surface and therefore its role as a membrane barrier, impeding the apical migration of epithelial and connective tissue cells, originating from the flap [15]. While these histologic data are certainly interesting, it appears reasonable to suggest that additional histologic studies are required to evaluate the “true” regenerative potential of PRP, which by no means has been proven as yet and represents a challenging issue for future research. Finally, in the future a higher number of RCTs should assess the clinical and radiographic efficacy of PRP as a monotherapy of periodontal endosseous defects.
Conclusion
Despite certain limitations inherent in a case report design (limited number of patients and, consequently, lack of a statistical analysis, as well as absence of a control group), the findings in the clinical cases we have reported support the following conclusions:
• PRP alone can achieve a clinically significant improvement in clinical parameters (clinical attachment level, probing pocket depth and bleeding on probing) and radiographic improvement at six months, compared with baseline, in periodontal endosseous defects of systemically healthy non-smoking (or lightly smoking) patients with generalized severe chronic periodontitis.
• The use of PRP alone may be clinically and radiographically efficacious in the treatment of periodontal endosseous defects.
Funding
None.
Conflicts of Interest
None
Article Info
Article Type
Case ReportPublication history
Received: Fri 09, Aug 2019Accepted: Mon 02, Sep 2019
Published: Mon 30, Sep 2019
Copyright
© 2023 Eudoxie Pepelassi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2019.04.02
Author Info
Eudoxie Pepelassi Harry Charalabos Stamatakis Ioannis Vrotsos Nikolaos Markou Sotirios Kotsovilis
Corresponding Author
Eudoxie PepelassiDepartment of Periodontology, School of Dentistry, National and Kapodistrian University of Athens, Athens, Greece
Figures & Tables
Table 1: Patient data for clinical cases 1 and 2.
|
CHARACTERISTIC |
CASE 1 |
CASE 2 |
|
Age (Years) |
54 |
55 |
|
Sex |
Male |
Male |
|
Smoking Status (Never, Former or Active Smoker; Daily Cigarette Consumption (Number); Smoking Duration (Years))* |
Never 0 0 |
Active 10 10 |
|
Full-Mouth Plaque Score (Percentage of Total Tooth Surfaces With Plaque)† Before scaling and root planing (SRP) Before surgery Final |
10% 8% 5% |
82%; 16%; 14% |
|
Gingival Score (Percentage of Total Tooth Surfaces With Bleeding)‡ Before SRP Before surgery Final |
11% 9% 6% |
75% 15% 12% |
|
Full-mouth Bleeding on Probing Score (Percentage of Total Tooth Surfaces With Bleeding on Probing)§ Before SRP Before surgery Final |
13% 10% 8% |
78% 19% 18% |
Abbreviations:
GI: Gingival index, according to Ainamo & Bay [22].
* Smoking data were recorded at the initial patient examination (before the patients underwent any therapy). For the patient in clinical case 1, smoking data remained unaltered through the final examination, whereas for the patient in clinical case 2, the final daily cigarette consumption was 10 cigarettes per day, i.e. was slighly reduced, compared with the initial value.
† Full-mouth plaque score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of plaque, when the clinician ran a periodontal probe tip on the tooth surface along the gingival margin. This score was a slight modification of the original “plaque control record” proposed by O’Leary and collegues, in which four aspects per tooth had been examined [22].
‡ Gingival score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of bleeding, when the clinician ran a periodontal probe tip on the tooth surface along the gingival margin (recorded according to the method of Ainamo and Bay) [23].
§ Full-mouth bleeding on probing score was recorded as the percentage of total tooth surfaces (six aspects per tooth) that revealed the presence of bleeding within 30 seconds after the clinician probed at the base (the most apical border) of each periodontal pocket.
Table 2: Defect data (baseline and final) for clinical case 1 (tooth no. 19).
|
TREATED TOOTH (ASPECT) / PARAMETER |
BASELINE DATA (Pre-surgical & Intra-surgical) |
FINAL DATA (POST-SURGICAL) |
|
Tooth no. 19 (Mesial) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
Clinical attachment level* (millimeters) |
10, 5, 13, 11, 5, 10 |
7, 5, 7, 8, 5, 7 |
|
Probing pocket depth* (mm) |
6, 2, 8, 6, 2, 6 |
3, 2, 3, 3, 2, 3 |
|
Gingival recession depth (apicocoronal)* (mm) |
4, 3, 5, 5, 3, 4 |
4, 3, 4, 5, 3, 4 |
|
Bleeding on probing* (present, +; absent, -) |
+, -, +, +, +, + |
-, -, -, -, -, + |
|
Furcation involvement† (no furcation defect, 0; furcation defect class, I, II or III) |
0, 0 |
0, 0 |
|
Mobility (no mobility, 0; mobility degree, 1, 2 or 3) |
1 |
1 |
|
Keratinized tissue width* (mm) |
3, 5, 4, 5, 4, 4 |
3, 5, 4, 5, 4, 4 |
|
Keratinized tissue thickness* (≥1 mm, +; <1 mm, -) |
+, +, +, +, +, + |
+, +, +, +, +, + |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
2 (facial and mesial) |
|
|
Tooth surfaces (number, location) |
1 (mesial) |
|
|
Distance from the acrylic stent to the alveolar crest‡ (mm) |
10, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base‡ (mm) |
15, 15 |
|
|
Distance from the alveolar crest to the endosseous defect base‡ (mm) |
5, 5 |
|
|
Width of the endosseous defect§ (mm) Faciopalatal Mesiodistal† |
9 5, 4 |
|
|
|
|
|
|
Tooth no. 19 (Distal) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
|
All pre-surgical data for the endosseous defect located distal to tooth no. 19 coincide with the pre-surgical data reported above for the endosseous defect located mesial to the same tooth |
All post-surgical data for the endosseous defect located distal to tooth no. 19 coincide with the post-surgical data reported above for the endosseous defect located mesial to the same tooth |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
2 (facial and distal) |
|
|
Tooth surfaces (number, location) |
3 (facial, distal and lingual) |
|
|
Distance from the acrylic stent to the alveolar crest¶ (mm) |
14, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base¶ (mm) |
18, 17 |
|
|
Distance from the alveolar crest to the endosseous defect base¶ (mm) |
4, 7 |
|
|
Width of the endosseous defect§ (mm) Faciopalatal Mesiodistal† |
9 5, 5 |
|
Abbreviations:
* Measurements at six sites per tooth, reported according to the sequence: mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual and disto-lingual.
† Measurements on two tooth aspects, reported according to the sequence: facial and lingual.
‡ Measurements at two sites per tooth (only mesial, because the endosseous defect was located mesially), reported according to the sequence: mesio-facial and mesio-lingual.
§ Measured at the level of the alveolar crest.
¶ Measurements at two sites per tooth (only distal, because the endosseous defect was located distally), reported according to the sequence: disto-facial and disto-lingual.
Table 3: Defect data (baseline and final) for clinical case 2 (tooth no. 28).
|
TREATED TOOTH (ASPECT) / PARAMETER |
BASELINE DATA (Pre-surgical & Intra-surgical) |
FINAL DATA (POST-SURGICAL) |
|
Tooth no. 28 (Mesial) |
|
|
|
|
|
|
|
|
Pre-surgical data |
|
|
Clinical attachment level* (millimeters) |
6, 4, 3, 5, 5, 3 |
6, 4, 3, 4, 3, 2 |
|
Probing pocket depth* (mm) |
4, 1, 3, 3, 3, 3 |
2, 1, 2, 0, 1, 1 |
|
Gingival recession depth (apicocoronal)* (mm) |
2, 3, 0, 2, 2, 0 |
4, 3, 1, 4, 2, 1 |
|
Bleeding on probing* (present, +; absent, -) |
+, -, -, +, -, - |
-, -, -, -, -, - |
|
Mobility (no mobility, 0; mobility degree, 1, 2 or 3) |
0 |
0 |
|
Keratinized tissue width* (mm) |
7, 2, 8, 7, 4, 8 |
7, 2, 8, 7, 4, 8 |
|
Keratinized tissue thickness* (≥1 mm, +; <1 mm, -) |
+, +, +, +, +, + |
+, +, +, +, +, + |
|
|
|
|
|
|
Intra-surgical data |
|
|
Osseous walls (number, location) |
Combination 1-2, mesial – (mesial and facial) |
|
|
Tooth surfaces (number, location) |
1 (mesial) |
|
|
Distance from the acrylic stent to the alveolar crest† (mm) |
10, 10 |
|
|
Distance from the acrylic stent to the endosseous defect base† (mm) |
13, 14 |
|
|
Distance from the alveolar crest to the endosseous defect base† (mm) |
3, 4 |
|
|
Width of the endosseous defect‡ (mm) Faciopalatal Mesiodistal§ |
5 3, 3 |
|
Abbreviations:
* Measurements at six sites, reported according to the sequence: mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual and disto-lingual.
† Measurements at two sites (only mesial, because the endosseous defect was located mesially), reported according to the sequence: mesio-facial and mesio-lingual.
‡ Measured at the level of the alveolar crest.
§ Measurements on two tooth aspects, reported according to the sequence: facial and lingual.

References
- Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55: 1294-1299. [Crossref]
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE et al. (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85: 638-646. [Crossref]
- Sánchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 18: 93-103. [Crossref]
- Kotsovilis S, Markou N, Pepelassi E, Nikolidakis D (2010) The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: a systematic review. J Periodontal Res 45: 428-443. [Crossref]
- Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I et al. (2007) Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol 34: 254-261. [Crossref]
- Okuda K, Tai H, Tanabe K, Suzuki H, Sato T et al. (2005) Platelet-rich plasma combined with a porous hydroxyapatite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. J Periodontol 76: 890-898. [Crossref]
- Demir B, Şengün D, Berberoğlu A (2007) Clinical evaluation of platelet-rich plasma and bioactive glass in the treatment of intra-bony defects. J Clin Periodontol 34: 709-715. [Crossref]
- Cheung WS, Griffin TJ (2004) A comparative study of root coverage with connective tissue and platelet concentrate grafts: 8-month results. J Periodontol 75: 1678-1687. [Crossref]
- Yen CA, Griffin TJ, Cheung WS, Chen J (2007) Effects of platelet concentrate on palatal wound healing after connective tissue graft harvesting. J Periodontol 78: 601-610. [Crossref]
- Simon D, Manuel S, Geetha V, Naik BR. Potential for osseous regeneration of platelet-rich plasma-a comparative study in mandibular third molar sockets. Indian J Dent Res 15: 133-136. [Crossref]
- Lindeboom JA, Mathura KR, Aartman IH, Kroon FH, Milstein DM et al. (2007) Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implants Res 18: 133-139. [Crossref]
- El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H et al. (2007) Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 78: 661-669. [Crossref]
- Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH (2008) Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res 19: 539-545. [Crossref]
- Trombelli L, Farina R (2008) Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J Clin Periodontol 35: 117-135. [Crossref]
- Keles GC, Cetinkaya BO, Baris S, Albayrak D, Simsek SB. Comparison of platelet pellet with or without guided tissue regeneration in the treatment of class II furcation defects in dogs. Clin Oral Investig 13: 393-400. [Crossref]
- Papli R, Chen S (2007) Surgical treatment of infrabony defects with autologous platelet concentrate or bioabsorbable barrier membrane: a prospective case series. J Periodontol 78: 185-193. [Crossref]
- Ilgenli T, Dündar N, Kal BI (2007) Demineralized freeze-dried bone allograft and platelet-rich plasma vs platelet-rich plasma alone in infrabony defects: a clinical and radiographic evaluation. Clin Oral Investig 11: 51-59. [Crossref]
- Pradeep AR, Shetty SK, Garg G, Pai S (2009) Clinical effectiveness of autologous platelet-rich plasma and peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol 80: 62-71. [Crossref]
- Markou N, Pepelassi E, Vavouraki H, Stamatakis HC, Nikolopoulos G et al. (2009) Treatment of periodontal endosseous defects with platelet-rich plasma alone or in combination with demineralized freeze-dried bone allograft: a comparative clinical trial. J Periodontol 80: 1911-1919. [Crossref]
- Pradeep AR, Pai S, Garg G, Devi P, Shetty SK (2009) A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol 36: 581-588. [Crossref]
- Markou N, Pepelassi E, Kotsovilis S, Vrotsos I, Vavouraki Het al. (2010) The use of platelet-rich plasma combined with demineralized freeze-dried bone allograft in the treatment of periodontal endosseous defects: a report of two clinical cases. J Am Dent Assoc 141: 967-978. [Crossref]
- O’Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43: 38. [Crossref]
- Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25: 229-235. [Crossref]
- Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4: 1-6. [Crossref]
- Nyman S, Lindhe J, Lundgren D (1975) The role of occlusion for the stability of fixed bridges in patients with reduced periodontal tissue support. J Clin Periodontol 2: 53-66. [Crossref]
- Needleman I, Moles DR, Worthington H (2005) Evidence-based Periodontology, systematic reviews and research quality. Periodontol 2000 37: 12-28. [Crossref]
- Laurell L, Gottlow J, Zybutz M, Persson R (1998) Treatment of intrabony defects by different surgical procedures. A literature review. J Periodontol 69: 303-313. [Crossref]
- Preshaw PM (2008) Host response modulation in periodontics. Periodontol 2000 48: 92-110. [Crossref]
