The Synergistic Effect of Anabolic Steroid and Loading on Intercellular Calcium Signaling Via Gap Junctions in Human Supraspinatus Tendon Cells
The Synergistic Effect of Anabolic Steroid and Loading on Intercellular Calcium Signaling Via Gap Junctions in Human Supraspinatus Tendon Cells
A B S T R A C T
Objective: We hypothesized that anabolic steroid administration would act synergistically with substrate strain in two-dimensional cultures of human supraspinatus tendon cells, to upregulate the expression of connexin-43 and to increase the Ca2+ wave propagation through gap junctions.
Methods: Supraspinatus tendon cells were isolated intra-operatively from human specimens during shoulder arthroscopy. Cells were plated in two-dimensional spot cultures and arranged into four experimental groups: 1) non-load, non-steroid (NLNS, n=12 wells); 2) non-load, steroid (NLS, n=12 wells); 3) load, non-steroid (LNS, n=12 wells); and 4) load, steroid (LS, n=12 wells) in order to produce bioartificial tendons (BATs). The load groups were stretched in culture plates and the steroid groups were given nandrolone decanoate. When BATs were macro- and microscopically mature, at five days, they were evaluated with immunocytochemistry for connexin-43 staining, fluorescence microscopy for calcium imaging and mechanical stimulation with a micropipette tip manipulation for calcium propagation. Dose response test was performed in order to establish any relation between nandrolone decanoate dose and calcium signaling response. ATP was applied to the spot culture cells from all groups and all patients to determine if the cells were sensitive to extracellular ATP.
Results: Load-steroid group demonstrated the greatest density of cnx43 in comparison to all other groups. There were no significant differences between the groups considering the percentage of cells responding after mechanical stimulation (cell recruitment). The cells of load-steroid group showed a significantly greater mean peak [Ca2+]ic compared to the values of the other groups (p<0.05). The propagation time was significantly decreased in the LS group compared with the other groups (p<0.05). There were no significant differences between the groups considering the number of cells that were responding spontaneously prior to stimulation or the number of responding cells that were oscillating after the stimulation.
Conclusion: Nandrolone decanoate and loading seem to have a synergistic effect on the upregulation of the gap junction protein cxn43 enhancing calcium signaling via gap junctions. Consecutively, anabolic steroid administration and load may enhance the formation of a better-organized cytoskeleton and particularly the actin stress monofilaments.
Keywords
Nandrolone decanoate, human supraspinatus tendon cells, cytomechanics, calcium signaling, connexins, gap junctions
Introduction
In all multicellular organisms, survival depends on an elaborate intercellular communication network that coordinates the growth, differentiation and metabolism of the multitude of cells in diverse tissues and organs. A common cell-to-cell communication pathway is the propagation of an intercellular Ca2+ signal [1]. Mechanical stimulation by micropipette indentation propagates a concentrically expanding Ca2+ signal [2]. This response has been observed in many cells’ types including tendon cells [3-9]. Intercellular Ca2+ signaling is mediated by several different mechanisms including the release or secretion of second messengers such as ATP, the intercellular diffusion of second messengers such as inositol triphosphate (IP3) and Ca2+ or a combination of these mechanisms [5, 10, 11]. Gap junctions are key components for the propagation of intercellular Ca2+ signals [11, 12].
Intercellular communication mediated by gap junctions plays an important role in a variety of cellular processes including cell differentiation, growth and proliferation, electrical activation of the heart and of smooth muscles, in neuronal signaling, but also in hormone secretion, auditory function, wound healing, lens transparency and immune functions [13]. Gap junctions are intercellular channels that connect the cytoplasm of two adjacent cells and thereby provide a direct diffusion pathway between the cells [1]. A gap junction is formed when a cylindrical hemichannel or connexon in one cell couples to another in an adjacent cell to create an aqueous pore between the two cells [13]. The connexon itself is composed of a hexameric assembly of proteins called connexins (Cxs). There are 21 different connexin genes identified in the human genome. Cxs can either form connexons by themselves or, in some cells, they can interact with different isoforms to form heteromeric connexons [14]. The permeability and gating characteristics of gap-junction channels depend on the connexin isoforms and on their post-translational modifications [13]. Cxs have been classified according to their predicted molecular weight and named accordingly. Connexin 43 (Cxn43), a 43kDa monomer, represents the most ubiquitous connexin and is important for the propagation of intercellular Ca2+ signals [1, 12, 15].
Tendons are connective tissue structures that connect muscles to bone and thus experience high tensile loading. Tendon cells have the potential to use direct cell-to-cell communication to detect and coordinate responses to load [16]. In vitro, tendon cells can propagate a Ca2+ wave via gap junctions in response to mechanical stimulation by micropipette indentation [9]. They express the gap junction protein cxn43 and modify its expression in response to load [17]. In vitro, tendon cells upregulate collagen and DNA synthesis 16 as well as cxs expression during cyclic tensile load, and inhibition of gap junction function leads to loss of this load response [16, 18]. Consequently, gap junctions coordinate the behavior of tendon cells in their key roles of load sensing and matrix modification.
Previous studies have shown that the expression of cxn43 has been changed during inflammatory states induced by various types of injury and that downregulation of cnx43 expression can be associated with cellular dysfunction and apoptosis [19-23].There are many reports in the literature about the deleterious effects of anabolic steroids on tendons, however, as most studies involve their systemic administration, it is extremely difficult to differentiate between presumable direct effects and the indirect effects exerted by the increased strain caused by the hypertrophied muscles [24-26].
Dihydrotestosterone, a potent testosterone metabolite, has been shown to exert direct effects in human cultured tenocytes, and it has been previously demonstrated that nandrolone decanoate acts synergistically with loading to increase matrix remodeling and the biomechanical properties of bioartificial tendons [27, 28].
We hypothesized that anabolic steroid administration would act synergistically with substrate strain in two-dimensional cultures of human supraspinatus tendon cells, to upregulate the expression of cnx43 and to increase the Ca2+ wave propagation through gap junctions.
Methods
I Tissue Harvesting
Partial samples of human supraspinatus tendons were harvested from the debrided tissue of four patients during surgical rotator cuff repair. Patients were all males with a mean age of 52 years (range= 34-66 years).
II Cell Isolation
Supraspinatus tendon cells were isolated from each specimen using a previously described technique [29]. The specimens were minced into small pieces with a sterile scalpel blade and rinsed with nutrient Medium 199 (GIBCO-Invitrogen Corp, New York, NY, USA) to remove red blood cells. The minced tendons were then digested with 0.1% collagenase in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) with antibiotics and HEPES, buffer pH 7.2 (GIBCO-Invitrogen Corp, New York, NY, USA) for 10 minutes at 37oC with gentle agitation, to disaggregate the sample and release the cells. Cells were plated at 25k cells/cm2 and grown to quiescence for 3-6 days until confluent. The cultures were kept at 37oC in a 5% CO2 humidified incubator and the media were changed every third day. Treatment with 0.05% trypsin-EDTA solution (Sigma-Aldrich, St. Louis, MO, USA) for 5 minutes was used to passage the cultures. Each initial cell culture was passaged 2-5 times to obtain an adequate sample for spot culture construction.
III Cell Culture
The cells were cultured in the Medium 199 with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mM ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), 15 mM sodium pyruvate (GIBCO-Invitrogen Corp, New York, NY, USA), 20 mM HEPES buffer, pH 7.2 (GIBCO-Invitrogen Corp, New York, NY, USA), Insulin-Transferrin-Sodium Selenite supplement (ITS) (Roche Diagnostics GmbH, Mannheim, Germany), and penicillin (100 units/ml)-streptomycin (100 μg/ml)-amphotericin-B (0.25 μg/ml) antibiotics (GIBCO-Invitrogen Corp, New York, NY, USA).
IV Spot-Cultures Preparation
Cells were plated in two-dimensional spot cultures at 2-3k cells per 10 μl spot in BioflexTM culture plates (Flexcell Int., Hillsborough, NC, USA). Each well contained two spots, and three wells were used for each treatment group per patient. The spot cultures were arranged into four experimental groups: 1) non-load, non-steroid (NLNS, n=12 wells); 2) non-load, steroid (NLS, n=12 wells); 3) load, non-steroid (LNS, n=12 wells); and 4) load, steroid (LS, n=12 wells). The cells remained in quiescence for 48 h post plating and then treated for five days.
V Anabolic Steroid Administration
After a 48-h quiescence period, where no anabolic agent was applied to the spot cultures, the steroid groups were given a final concentration of 100 nM nandrolone decanoate (Deca-Durabolin 200 mg/ml, Organon Inc.). Results of dilution studies were used to determine this dosage that was equivalent to the clinical prescription dose. The drug contained 200 mg nandrolone decanoate USP per ml of sterile sesame oil with 5% benzyl alcohol as preservative. The stock drug-solution was diluted 1:1 with sterile dimethylsulfoxide (DMSO). The steroid solution was made fresh daily, diluted in culture medium to 100 nM and dispensed into the culture medium. Control cultures received vehicle alone of the appropriate dilution.
VI Loading
The FX3000 FlexerCell Strain UnitTM (Flexcell Int., Hillsborough, NC, USA) was used for stretching the cells. The Bioflex TM culture plate consisted of a six-well culture plate where each well had a collagen-coated rubber growth surface. The plates were placed atop a vacuum manifold baseplate with surrounding rubber gaskets, and vacuum applied to the undersurface of each well, deforming the rubber substrate downwards to stretch it. Cultures were thus exposed to biaxial strain. The FX3000 computer controlled the intensity and duration of vacuum 1% equibiaxial strain at 1 Hz in a regime of 1 hour on, 23 hours resting, for 5 days. Media were changed daily 1 hour prior to stretching.
VII Immunocytochemistry
For cxn43 staining, cells were rinsed twice in PBS and fixed with 3.7% formaldehyde at room temperature for 15 min. Then, the fixed cells were permeabilized with 0.2% Triton X-100 in PBS containing 0.5% bovine serum albumin (BSA) (Sigma - Aldrich, St. Louis, MO, USA) and kept in dark, at room temperature, for 40 min. Cells were incubated with rabbit anti-connexin 43 antibody (Zymed Laboratories Inc, San Francisco, CA, USA)) (dilution 1:100) at room temperature, for 2 hours. Then, cells were incubated with rhodamine-redTM - X goat anti - rabbit IgG (H + L) antibody (Molecular Probes Inc, Eugene, OR, USA) (dilution 1:500) in the blocking buffer, at room temperature, for 1 hour. All cells were mounted on a glass slide in a mounting medium (Biomeda Corp., Foster City, CA, USA). The preparations were examined using the Olympus BX60F5 microscope equipped with an Olympus FV12 digital camera and an Olympus MicroSuiteTM B3SV image capture software (Olympus Optical Co, Japan).
VIII Calcium Imaging
On day five of treatment, cultures were rinsed twice with Earles’ Balanced Salt Solution (EBSS), loaded with 5 μM fura-2AM, a calcium fluorescent indicator (Molecular Probes Inc, Eugene, OR, USA) and incubated in the dark for 45 minutes at room temperature. Cultures were then rinsed twice with EBSS to remove residual fura-2AM. The silicone membranes in the wells of the BioflexTM plates excised with a scalpel and mounted in an 83 mm diameter plastic culture plate on the stage of the microscope. An Olympus BX- 51TRF upright fluorescence microscope (Olympus Optical Co, Japan) was used, equipped with a Lambda DG- 4 wavelength switcher and light guide (Sutter Instruments Corp, Novato, CA, USA), a CoolSNAP-fx CCD camera (Photometrics, Tucson, AZ, USA) and an Isee analytical imaging software (Inovis, Inc.) The cells were subjected to mechanical stimulation by micropipette indentation and intracellular calcium concentration was quantitated. Intracellular calcium levels were measured using a ratio imaging method, which entails exciting cells alternately at 340 and 380 nm observing emitted fluorescence at 510 nm and above and comparing ratios to known Ca2+ standards. Images were subjected to background subtraction to correct for non-uniformity across the fluorescence field. Basal Ca2+ values were obtained for 60 sec prior to mechanical stimulation.
IX Mechanical Stimulation
Single cells were mechanically stimulated by transient deformation of the membrane using a glass micropipette (tip diameter about 1μm) mounted on an MX7600L motorized manipulator (Siskiyou Design Instruments, Grants Pass, OR, USA) and guided by computer software (Siskiyou Design Instruments, Grants Pass, OR, USA).
X Dose Response Test
Dose response test was performed in order to establish any relation between nandrolone decanoate dose and calcium signaling response. Spot cultures from all groups and patients were prepared as previously mentioned and cells were stimulated by the addition of 1, 10, 100, and 1000 nM nandrolone decanoate final concentrations diluted in EBSS. Cells were stimulated with nandrolone decanoate once and the above described ratio-imaging technique was used to quantitate the [Ca2+]ic.
XI ATP Test
ATP was applied to the spot culture cells from all groups and all patients to determine if the cells were sensitive to extracellular ATP. Cells were stimulated by the addition of 1 μM ATP final concentration diluted in EBSS. The appropriate concentration of ATP for stimulation was determined from an ATP dose response experiment with concentrations starting at 0.1 μM and increasing to 100 μM. Cells were stimulated with ATP once and the above described ratio-imaging technique was used to quantitate the [Ca2+]ic.
XII Calcium Signaling Parameters
Analyzing the calcium signaling data, many parameters were calculated: a) cell recruitment, b) mean peak [Ca2+]ic, c) mean average response, d) propagation time, e) response rate, e) spontaneously responding cells, and f) oscillating cells. All data were normalized to the mean basal level of [Ca2+]ic to account for the variations from daily set up errors in light intensity and in the amount of Fura-2AM uptake. The basal level was determined by averaging the [Ca2+]ic of the prior to mechanical stimulation condition over 60 sec. A responding cell was mathematically defined as a cell that increased its [Ca2+]ic four standard deviations over its basal level.
Cell recruitment was expressed by the percentage of cells responded to mechanical stimulation. This percentage was calculated by dividing the number of responding cells by the total number of cells of each trial. The mean peak [Ca2+]ic referred to the average of the maximum [Ca2+]ic post-stimulation for all responding cells. The mean average response for each cell referred to the [Ca2+]ic measured every 1 sec and averaged from the time of initial rise until the return to basal level post-stimulation. The propagation time was determined, as the time required propagating a Ca2+ wave from a stimulated cell to an adjacent unstimulated cell. The stimulated cell is defined as the first responding cell at the time of mechanical stimulation. The time of arrival was defined as the time when the slope of the [Ca2+]ic line of the cell changed from baseline to a new increase. The response rate of each cell was measured from the slope of the linear part of the [Ca2+]ic graph. The mean response rate of each trial expresses the rate of intracellular calcium increase of each group. A spontaneous response was determined as the transient increase in [Ca2+]ic prior to mechanical stimulation and considered to be a non-mechanically response. Ca2+ oscillations were defined as post-stimulation repetitive intracellular Ca2+ waves demonstrating a synchronous or asynchronous increase and decrease of the [Ca2+]ic [30-35].
XII Statistical Analysis
Statistical analysis of data was performed using SigmaStat statistical software program (SPSS Science, Chicago, IL, USA). Level of statistical significance was set at p<0.05 and determined with a One-Way ANOVA and a Student’s t-test. Data sets are representative of each patient cell isolate.
Results
I Immunocytochemistry
All cell processes of all groups had bright green colored foci of immunolabel for cnx43 localized at the contact areas with other cells indicating the presence of gap junctions. The foci were clearly visible between cell bodies and between cell processes. Cnx43 appeared to be more prominent towards the cell processes. LS group demonstrated the greatest density of cnx43 in comparison to the other groups.
II Calcium Signaling
All concentrations of nandrolone decanoate induced an increase in [Ca2+]ic when compared to basal values. The greatest and fastest increase was observed between 100 and 1000 nM nandrolone decanoate; therefore 100 nM nandrolone decanoate was chosen for testing the response of cells. Also, the ATP test was positive for all groups and all patients rendering the human supraspinatus tendon cells capable to increasing their [Ca2+]ic after stimuli. This observation allowed us to consider these cell cultures appropriate for our study.
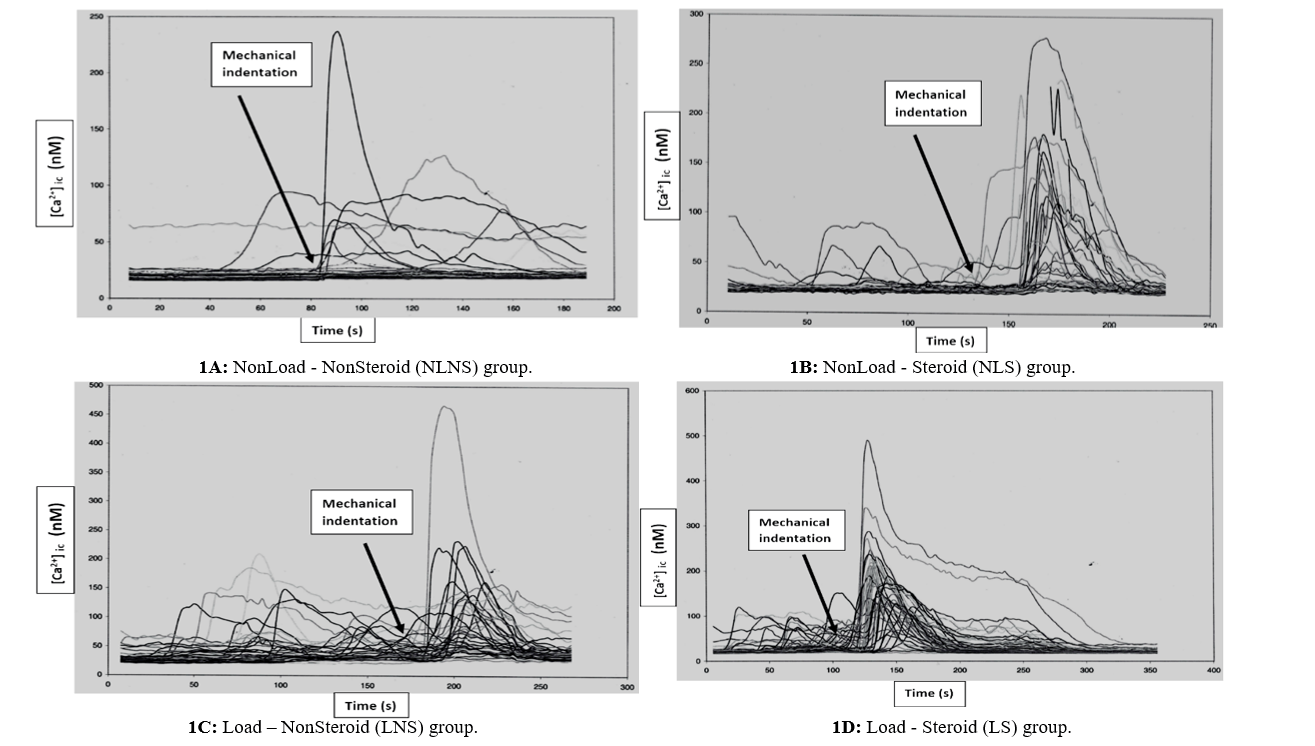
Figure 1: A-D) Mechanical stimulation by micropipette indentation, after a period of cell quiescence, induces a calcium wave that propagates to adjacent tendon cells. Once the cells are chemically stimulated there is a rapid increase in [Ca2+]ic. Each line represents an individual cell’s response to stimulation.
There were no significant differences between the groups considering the percentage of cells responding after mechanical stimulation (cell recruitment). The cells of LS group showed a significantly greater mean peak [Ca2+]ic compared to the values of the other groups (p<0.05) (Figure 1). Also, LNS group showed significantly greater mean peak [Ca2+]ic compared to the values of NLNS group (p=0.026). The propagation time was significantly decreased in the LS group compared with the other groups (p<0.05). The NLNS group showed increased time of Ca2+ wave propagation while loading alone (LNS group) and anabolic steroid administration alone (NLS group) decreased the time when acting independently. However, the differences between NLNS, NLS and LNS groups considering the propagation time were not statistically significant. There were no significant differences between the groups considering the number of cells that were responding spontaneously prior to stimulation or the number of responding cells that were oscillating after the stimulation.
Discussion
Physiotherapy protocols after tendon injury or repair are designed to improve healing, reduce adhesion formation and increase range of motion of the involved joint. Manipulation of the mechanical environment of the healing tendon may exert a biologic effect through the mechanotransduction mechanism and multiple studies have demonstrated that eccentric exercise programs are effective for the treatment of tendinopathy [36].
Parallel to motion therapy, on molecular and biochemical level, processes such as cell migration and division and matrix synthesis must provide a biomechanically sound tendon [37]. To coordinate these processes, mechanical and chemical signals must be communicated between cells through a variety of pathways utilizing mechanosensory complexes and second messengers [38, 39]. Calcium (Ca2+) is one of the primary second messengers utilized by cells to transduce mechanical to biochemical signals via gap junctions regulating multiple cell functions including gene transcription, cell growth and proliferation and apoptosis [13].
The primary purpose of our study was to determine the effect of anabolic steroid administration and stretching on the intercellular calcium signaling via gap junctions. Given that gap junctions couple tendon cells electrically and chemically and coordinate responses to mechanical load, the administration of anabolic steroid may alter their role on detecting damages or allowing metabolic cooperation between cells [16, 40]. An increase of gap junction protein expression and intercellular communication could result in positive attributes after tendon injury.
There are no data about the role of connexins and gap-junctional intercellular communication in tendon healing, even though Cnx43 has been shown to play an important role in healing, yet with somewhat contradictory findings [41, 42]. In our study, cxn43 appeared to be more prominent towards the cell processes. This finding is in accordance with the findings of McNeilly and co-authors who studied the distribution of cxn43 and cxn32 in rat’s deep flexor tendons [43]. They found that cxn43 was associated with cell processes while cxn32 was associated with junctions between cell bodies rather than cell processes. The authors suggested that given the different characteristics and distributions of cxn43 and cxn32 between tendons, it could be that the two different types of junction might be responsible for sensing two different forms of loading.
We noticed that the cultures of LS group demonstrated the greatest density of cxn43 in comparison to the other groups. It seems that anabolic steroid administration and load enhance cnx43 expression in a complementary manner. Studies about the effects of mechanical strain on the expression of cnx43 have reported conflicting results, which may be either attributed to intrinsic differences between various tissues or to variations in the amount and type of strain used [17, 44-46]. In agreement with the latter speculation is the study by Maeda et al. The application of two different levels of static tensile strain in tenocytes isolated from Achilles tendons of Japanese white rabbits resulted in different effects in gap junction intercellular communication [47]. 4% strain resulted in enhanced gap junction intercellular communication primarily attributed to the opening of gap junction pores and accompanied by a relocation of the expression of cnx43 within the cytoplasm and near the cell nuclei, while the application of higher strain (8%) significantly reduced gap junction intercellular communication and was accompanied by reduced immunofluorescence staining for cnx43. Similarly, the application of static tensile load might differentially affect the expression of cnx43 depending on the duration of the load [17].
On the other hand, androgens have been found to alter the expression of cxn43 in tissues such as the prostate and ovaries and thus affect cell to cell communication [48, 49]. In our study, the combination of loading and steroid administration (LS) resulted in enhanced measures of intercellular communication as expressed by greater mean peak [Ca2+]ic and decreased propagation time. This could be associated either with increased connexin expression or with changes in gap junction permeability. Our results indicate that the enhanced intercellular communication was associated with greatest density of cnx43 at the cell processes. This was not observed in the study by Maeda et al. where the enhanced gap junction intercellular communication was related to the opening of gap junction pores [47].
Several studies have demonstrated an interactive relationship between connexins and the actin cytoskeleton [50-53]. Functional blocking of gap junctions resulted in discordance of actin stress fibers between neighboring cells. Mechanical stimulation-induced calcium wave propagation was significantly reduced in these cells [54]. A similar association between actin and cnx43 has been described in avian and human tenocytes as well [55]. Moreover, this association seems to increase after cyclic stain and might correlate with the degree of strain. In our study anabolic steroid administration and load led to upregulation of cxn43 and to a greater number of gap junctions in comparison with the other groups. Consecutively, anabolic steroid administration and load may enhance the formation of a better-organized cytoskeleton and particularly the actin stress monofilaments. Notably, it is previously described that nandrolone administration has led to a better organized actin cytoskeleton [28].
There are many reports in the literature about the detrimental effects of anabolic steroids on tendon cells [24-26]. However, these studies are confounded by the inevitable effects of anabolic steroids on muscles and the increase in muscle force associated with chronic administration. A few studies have assessed the direct effects of anabolic steroids on tendon cells. In vitro, dihydrotestosterone has been shown to increase the proliferation of human tenocytes from intact supraspinatus tendons after 48h and 72h treatment and to alter their morphology [27]. The proliferation rates however were similar between treated tenocytes and controls at 96h advocating an acute and provisional effect. We have previously demonstrated that the administration of nandrolone decanoate in bioartificial tendons has resulted in increased remodeling, a more organized cytoskeleton and improved biomechanical properties [28].
There are limited data about the effects of anabolic steroids on the musculotendinous unit after injury. The administration of anabolic steroids seems to protect against muscle damage caused by rotator cuff tendon release, by inhibiting the fatty infiltration that usually follows after such injuries [56, 57]. Notably, this effect might be influenced by the timing of administration, since once the fatty infiltration has been established, anabolic steroids are unable to reverse the damage [58]. However, administration of nandrolone decanoate locally on the rotator cuff after incision and reconstruction surgery exerted negative effects inhibiting the healing process [59]. It is possible that, just as with mechanical load, the dose of anabolic steroids, as well as the timing of administration might influence their effect on tendon healing.
In conclusion, we demonstrated that nandrolone decanoate and loading seem to have a synergistic effect on the upregulation of the gap junction protein cxn43 enhancing calcium signaling via gap junctions. The importance of mechanical stimulation and the anabolic effect of biological or pharmaceutical factors in normal homeostasis of tissues during post-injury repairing process may be integral in advancing therapeutic approaches and motion therapy regimens. Further investigations are needed to establish the role of anabolic steroids in conjunction with motion therapy in the management of complex and challenging tendon injury cases.
Limitations
There were some limitations considering our culture mechanostimulus instrumentation. The state of strain achieved varied as a function of position on the substrate culture surface (strain heterogeneity). Also, at any given site on the substrate culture surface, the state of strain varies as a function of direction (strain anisotropy). Moreover, motion of the substrate induces motion of the overlying liquid nutrient medium. This motion exerts unintended reactive stresses upon the culture layer [60]. Finally, the loading regime was only 1% stretch at 1 Hz for only 1h per day for 5 days. That regime may seem to be less aggressive than other applied and consecutively less effective. However, it is demonstrated that acute or excessive load breaks gap junction. Excessive strain greater than 5% cell elongation at 1 Hz for 3-7 days leads to smaller and less numerous cxn43 than non-stretched cells. Also, stretching cells discontinuously, allowing at least 18h of non-load stimulates connexin protein expression [61].
Article Info
Article Type
Research ArticlePublication history
Received: Sat 18, Apr 2020Accepted: Mon 04, May 2020
Published: Fri 08, May 2020
Copyright
© 2023 Ioannis K. Triantafyllopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2020.01.05
Author Info
Ioannis K. Triantafyllopoulos Natasa Dede
Corresponding Author
Ioannis K. TriantafyllopoulosLaboratory for the Research of Musculoskeletal Disorders, Medical School, National & Kapodistrian University of Athens, Greece
Figures & Tables
References
- Leybaert L, Sanderson MJ (2012) Intercellular Ca(2+) waves: mechanisms and function. Physiol Rev 92: 1359-1392. [Crossref]
- Wall ME, Banes AJ (2005) Early responses to mechanical loading in tendon: role of calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact 5: 70-84. [Crossref]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ (1991) Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6: 983-992. [Crossref]
- Abu Khamidakh AE, Juuti Uusitalo K, Larsson K, Skottman H, Hyttinen J (2013) Intercellular Ca(2+) wave propagation in human retinal pigment epithelium cells induced by mechanical stimulation. Exp Eye Res 108: 129-139. [Crossref]
- Ryu SY, Peixoto PM, Won JH, Yule DI, Kinnally KW (2010) Extracellular ATP and P2Y2 receptors mediate intercellular Ca(2+) waves induced by mechanical stimulation in submandibular gland cells: Role of mitochondrial regulation of store operated Ca(2+) entry. Cell Calcium 47: 65-76. [Crossref]
- Halidi N, Boittin FX, Bény JL, Meister JJ (2011) Propagation of fast and slow intercellular Ca(2+) waves in primary cultured arterial smooth muscle cells. Cell Calcium 50: 459-467. [Crossref]
- Furuya K, Enomoto K, Maeno T, Yamagishi S (1993) Mechanically induced calcium signal in mammary epithelial cells. Jpn J Physiol 43: S105-S108. [Crossref]
- Sanderson MJ, Charles AC, Dirksen ER (1990) Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul 1: 585-596. [Crossref]
- Kenamond CA, Weinhold P, Bynum DK, Tsuzaki M, Benjamin M et al. (1997) Human tendon cell express connexin-43 and propagate a calcium wave in response to mechanical stimulation [abstract]. Transactions of the 43rd Annual Meeting of Orthopaedic Research Society 22: 179.
- Zimmerman B, Walz B (1999) The mechanism mediating regenerative intercellular Ca2+ waves in the blowfly salivary gland. EMBO J 18: 3222-3231. [Crossref]
- Boitano S, Dirksen ER, Sanderson MJ (1992) Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258: 292-295. [Crossref]
- Halidi N, Alonso F, Burt JM, Bény JL, Haefliger JA et al. (2012) Intercellular calcium waves in primary cultured rat mesenteric smooth muscle cells are mediated by connexin43. Cell Commun Adhes 19: 25-37. [Crossref]
- Hervé JC, Derangeon M (2013) Gap-junction-mediated cell-to-cell communication. Cell Tissue Res 352: 21-31. [Crossref]
- Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M et al. (2012) Gap junctions. Compr Physiol 2: 1981-2035. [Crossref]
- Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H et al. (1997) Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. J Neurosci Res 49: 528-540. [Crossref]
- Banes AJ, Weinhold P, Yanh X, Tsuzaki M, Bynum D et al. (1999) Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop Relat Res 367: S356-S370. [Crossref]
- Maeda E, Ye S, Wang W, Bader DL, Knight MM et al. (2012) Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech Model Mechanobiol 11: 439-447. [Crossref]
- Waggett AD, Benjamin M, Ralphs JR (1999) Gap junction inhibitors abolish strain response in tendon cells in vitro. Trans Orthop Res Soc 24: 360.
- Kimura K, Orita T, Morishige N, Nishida T, Sonoda KH (2013) Role of the JNK signaling pathway in downregulation of connexin43 by TNF-α in human corneal fibroblasts. Curr Eye Res 38: 926-932. [Crossref]
- Fernandez Cobo M, Gingalewski C, Drujan D, De Maio A (1999) Downregulation of connexin 43 gene expression in rat heart during inflammation. The role of tumour necrosis factor. Cytokine 11: 216-224. [Crossref]
- Brand Schieber E, Werner P, Iacobas DA, Iacobas S, Beelitz M et al. (2005) Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J Neurosci Res 80: 798-808. [Crossref]
- Li AF, Roy S (2009) High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci 50: 1400-1407. [Crossref]
- Tien T, Muto T, Barrette K, Challyandra L, Roy S (2014) Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Mol Vis 20: 732-741. [Crossref]
- Tsitsilonis S, Chatzistergos PE, Mitousoudis AS, Kourkoulis SK, Vlachos IS et al. (2014) Anabolic androgenic steroids reverse the beneficial effect of exercise on tendon biomechanics: an experimental study. Foot Ankle Surg 20: 94-99. [Crossref]
- Marqueti RC, Prestes J, Wang CC, Ramos OH, Perez SE et al. (2011) Biomechanical responses of different rat tendons to nandrolone decanoate and load exercise. Scand J Med Sci Sports 21: e91-e99. [Crossref]
- Seynnes OR, Kamandulis S, Kairaitis R, Helland C, Campbell EL et al. (2013) Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties. J Appl Physiol (1985) 115: 84-89. [Crossref]
- Denaro V, Ruzzini L, Longo UG, Franceschi F, De Paola B et al. (2010) Effect of dihydrotestosterone on cultured human tenocytes from intact supraspinatus tendon. Knee Surg Sports Traumatol Arthrosc 18: 971-976. [Crossref]
- Triantafillopoulos IK, Banes AJ, Bowman KF Jr, Maloney M, Garrett WE Jr et al. (2004) Nandrolone decanoate and load increase remodeling and strength in human supraspinatus bioartificial tendons. Am J Sports Med 32: 934-943. [Crossref]
- Banes AJ, Donlon K, Link GW, Gillespie Y, Bevin AG et al. (1988) Cell populations of tendon: a simplified method for isolation of synovial and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res 6: 83-94. [Crossref]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM et al. (1998) Differential effect of steady versus oscillating flow on bone cells. J Biomech 31: 969-976. [Crossref]
- Jaffe L (2006) The discovery of calcium waves. Semin Cell Div Biol 17: 229. [Crossref]
- Kawano S, Otsa K, Shoj S, Yamagata K, Hiraoka M (2003) Ca2+ oscillations regulated by Na+-Ca2+ exchanger and plasma membrane Ca2+ pump induce fluctuations of membrane currents and potentials in human mesenchymal stem cells. Cel Calcium 34: 145-156. [Crossref]
- Thomas D, Mason MJ, Mahaut Smith MP (2001) Depolarisation-evoked Ca2+ waves in the non-excitable rat megakaryocyte. J Physiol 537: 371-378. [Crossref]
- Sage SO, Yamoah EH, Heemskerk JW (2000) The roles of P(2x1) and P(2T AC) receptors in ADP-evoked calcium signaling in human platelets. Cell Calcium 28: 119-126. [Crossref]
- Gelberman RH, Steinberg D, Amiel D, Akeson W (1991) Fibroblast chemotaxis after tendon repair. J Hand Surg Am 16: 686-693. [Crossref]
- Galloway MT, Lalley AL, Shearn JT (2013) The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am 95: 1620-1628. [Crossref]
- Killian ML, Cavinatto L, Galatz LM, Thomopoulos S (2012) The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 21: 228-237. [Crossref]
- Banes AJ, Tsuzaki M, Yamamoto J, Fischer T, Brigman B et al. (1995) Mechanoreception at the cellular level the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol 73: 349-365. [Crossref]
- Banes AJ, Tsuzaki M, Elfervig M, Qi J (2001) Mechanical forces and signaling in connective tissue cells: cellular mechanisms of detection, transduction, and responses to mechanical deformation. Curr Opin Orthop 12: 386-396.
- Ralphs JR, Waggett AD, Benjamin M (2002) Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol 21: 67-74. [Crossref]
- Churko JM, Shao Q, Gong XQ, Swoboda KJ, Bai D et al. (2011) Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects. Hum Mutat 32: 456-466. [Crossref]
- Qiu C, Coutinho P, Frank S, Franke S, Law LY et al. (2003)Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 13: 1697-1703. [Crossref]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR (1996) Tendon cells in vivo form a three-dimensional network of cell processes by gap junctions. J Anat 189: 593-600. [Crossref]
- Cowan DB, Lye SJ, Langille BL (1998) Regulation of vascular connexion 43 gene expression by mechanical loads. Circ Res 82: 786-793. [Crossref]
- Bupha Intr T, Haizlip KM, Janssen PM (2009) Temporal changes in expression of connexin 43 after load-induced hypertrophy in vitro. Am J Physiol Heart Circ Physiol 296: H806-H814. [Crossref]
- Zhuang J, Yamada KA, Saffitz JE, Kléber AG (2000) Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res 87: 316-322. [Crossref]
- Maeda E, Ohashi T (2015) Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem Biophys Res Commun 465: 281-286. [Crossref]
- Huynh HT, Alpert L, Laird DW, Batist G, Chalifour L et al. (2001) Regulation of the gap junction connexin 43 gene by androgens in the prostate. J Mol Endocrinol 26: 1-10. [Crossref]
- Yang M, Li J, An Y, Zhang S (2015) Effects of androgen on immunohistochemical localization of androgen receptor and Connexin 43 in mouse ovary. Tissue Cell 47: 526-32. [Crossref]
- Cotrina ML, Lin JH C, Nedergaard M (1998) Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci 18: 8794-8804. [Crossref]
- Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS et al. (2012) Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res 110: 978-989. [Crossref]
- Crespin S, Bechberger J, Mesnil M, Naus CC, Sin WC (2010) The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J Cell Biochem 110: 589-597. [Crossref]
- Tadvalkar G, Pinto da Silva P (1983) In vitro rapid assembly of gap junctions by cytoskeleton disruptors. J Cell Biol 96: 1279-1287. [Crossref]
- Yamane Y, Shiga A, Asou H, Ito E (2002) GAP junctional channel inhibition alters actin organization and calcium propagation in rat cultured astrocytes. Neuroscience 112: 593-603. [Crossref]
- Wall ME, Otey C, Qi J, Banes AJ (2007) Connexin 43 is localized with actin in tenocytes. Cell Motil Cytoskeleton 64: 121-130. [Crossref]
- Gerber C, Meyer DC, Nuss KM, Farshad M (2011) Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J Bone Joint Surg Am 93: 2189-2195. [Crossref]
- Gerber C, Meyer DC, Flück M, Benn MC, von Rechenberg B et al. (2015) Anabolic Steroids Reduce Muscle Degeneration Associated With Rotator Cuff Tendon Release in Sheep. Am J Sports Med 43: 2393-2400. [Crossref]
- Gerber C, Meyer DC, Von Rechenberg B, Hoppeler H, Frigg R et al. (2012) Rotator cuff muscles lose responsiveness to anabolic steroids after tendon tear and musculotendinous retraction: an experimental study in sheep. Am J Sports Med 40: 2454-2461. [Crossref]
- Papaspiliopoulos A, Papaparaskeva K, Papadopoulou E, Feroussis J, Papalois A et al. (2010) The effect of local use of nandrolone decanoate on rotator cuff repair in rabbits. J Invest Surg 23: 204-207. [Crossref]
- Brown EM, McLeod RJ (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239-297. [Crossref]
- Banes AJ, Horesovsky G, Tsuzaki M, Boitano S, Lawrence WT et al. (1999) The connexin 43 gap junction is a mechanosensitive gene in avian flexor tendon cells. In: Caterson B, Archer C, Benjamin M, Ralphs J (eds) The biology of the synovial joint. Harwood Academic Publishers, Amsterdam, Netherlands 1999: 279-299.
