Journals
The Significance of p53 and Estrogen Expression in Endometrial Hyperplasias and Endometrioid Carcinomas: How should we evaluate immunohistochemistry?
A B S T R A C T
Objective: Endometrial carcinomas are the most commonly encountered malignancies of the female genital system. The tumours are sub-classified into two types; type I, endometrioid carcinoma (EC) and type II, serous carcinoma (SC). Accumulation of p53 protein have been detected in SC whereas they are not common in endometrial hyperplasia and EC.
Materials-Methods: This study is a retrospective evaluation of 51 endometrioid carcinomas, 46 endometrial hyperplasias with/without atypia. In all three groups, the correlations between epithelial/ stromal p53 and estrogen expression (ER) and cervical, myometrial and lymphovascular invasion and relationship tumour grades were determined.
Results: Significant differences were noted between hyperplasia with atypia and non-hyperplasic endometria, epithelial p53 immunostaining (p < 0.05). ER expression were stronger in hyperplasias with/without atypia than EC in both epithelial and stromal components (p < 0.05). The relationship between tumour grade and p53 and ERs epithelial/ stromal expression was investigated however no statistically significant correlation was found.
Conclusion: The present study found that, most EC cases have a higher p53 but lower ER expression. P53 expression can be used as an indicator of tumour aggressiveness. In addition to known factors that need to be considered when evaluating endometrioid carcinomas and their precursor lesions, first glance importance should be given to epithelial expression of p53 and stromal expression of ER.
K E Y W O R D S
endometrial hyperplasia, p53 expression, estrogen expression, endometrioid carcinoma
I N T R O D U C T I O N
Endometrial carcinomas are the most common malignant tumours of the female genital tract. The tumours are sub-classified into two types; type I, EC and type II, SC. This classification is based on epidemiological, clinical and pathological features and molecular findings. SC account for a minority of endometrial carcinomas and do not seem to be associated with estrogenic risk factors. In contrast, EC, the most common type, is considered to be related with excess estrogen exposure [1].
In addition, mutations in the p53 gene and accumulation of p53 protein have been detected in SC whereas they are not common in EC and endometrial hyperplasia [2,3]. Distinct carcinogenic pathways have been described in each subtype. Type I, are characterized by microsatellite instability and alterations of the PTEN, KRAS genes whereas type II SC often show over expression of p53 and Her2/neu [3].
However, it has been previously suggested that, a progression in molecular alterations from grade 1 to grade 3 EC exists. The frequency of p53 over expression in EC reported has ranged from 14% to 25% in low grade ECs and from 2.5% to 69% in grade 3 ECs [4]. It has been reported that grade 3 ECs share molecular pathway with SCs. In addition, p53 staining in ECs has been described as a sign of p53 genetic heterogeneity [5].
On the other hand, ER are direct promoters of type I endometrial carcinogenesis. ER are overexpressed in both hyperplasia and carcinoma, as well as in epithelial and stromal cell populations [6,7]. ER and progesterone receptors generally show concordant expression and their functional status may influence the development of endometrial carcinoma [8].
M a t e r i a l m e t h o d s
A total of 51 cases of EC and 46 cases of endometrial hyperplasia were included in this study. Of these cases, 29 were hyperplasia with atypia, 17 were hyperplasia without atypia, according to the WHO 2014 classification system (Silverberg 2014). Fifty-one cases of ECs were assessed by two blinded pathologists regarding tumour grade, lymphovascular invasion, cervical and myometrial invasion and non-tumoral endometrial tissues.
Immunohistochemical antibodies of p53 (Mouse monoclonal antibody, Clone DO-7,Leica, Novocastra,diluted 1:50) and Estrogen Receptor (Rabbit monoclonal antibody, Clone SP-1,Biocare, diluted 1:100) were studied using an automated immunohistochemistry stainingdevice (Ventana BenchMark AutoStainer). Colon adenocarcinoma for p53, and breast tissue for estrogen were included as external positive controls. Omission of the primary antibody was used as negative controls. For epithelial and stromal assessment, nuclear staining intensity of p53 and ER was scored using three categories; mild, moderate and strong. The staining ratio was scored as 0 for no staining, 1 for < 10%, 2 for 10% to 50% and 3 for >50%.
The data were summarized as frequencies and percentages. The categorical variables were analyzed using Pearson Chi-Square test with exact method or Mann Whitney-U test. The mean age of the patients was assessed using Student's t test. p< 0.05 values were accepted as significant. Statistical calculations were done by IBM SPSS Statistics 23.0 for Windows 7.
R e s u l t s
Ages of patients with EC ranged from 36 to 87 (mean 57.2) years. The 51 EC's cases with hyperplastic adjacent endometrium comprised of 18 with atypical hyperplasia and 3 with hyperplasia without atypia and 18 with nonhyperplasic endometria. The remaining 12 cases did not reveal non-tumoral endometrial areas on the sections. Sixteen cases (31.3%) were FIGO grade 1 EC, 29 cases (56.8%) were grade 2 and 7 cases (11.7%) were grade 3. All patients were evaluated for tumour size, cervical and myometrial invasion and the presence of lymphovascular invasion.
Cervical invasion was described in four cases (9.3%), the myometrial invasion showed over 50% involvement (TNM-T1b) in 11 cases (25.5%) and lymphovascular invasion was detected in 11cases (25.5%). In twentyfive cases (58.1%) tumour sizes were less than 4 centimetres (In eight cases diagnosis were made by probe curettage). p53 expression was reduced in both stromal and epithelial components of non-tumoral endometria compared with EC (Figure 1A). No significant differences were noted between non-hyperplastic endometrium and hyperplasia with atypia by stromal p53 immunostaining (p>0.05) (Figure 1A,1E). But significant differences were noted between hyperplasia with atypia and nonhyperplasic endometria, by epithelial p53 immunostaining (p < 0.05) (Figure 1A,1E). ER had a stronger expression in both epithelial and stromal components of hyperplasias with/without atypia (Figure 1D,1F). ER were stronger positive in hyperplasias with/without atypia than EC in both epithelial and stromal components (p < 0.05). Similarly ER expression showed significant differences in hyperplastic endometria both in epithelial and stromal components compared to EC (p < 0.05) (Figure 1B, 1J).
The relationship between tumour grade and both epithelial and stromal expression of p53 was investigated however no statistically significant correlation was found (p>0.05) (Figure 1G,1I). In a similar fashion, tumour grade and epithelial ER expression did not show any correlation. A positive correlation between tumour grade and stromal ER expression was noted (p < 0.05) (Figure 1H,1J) . There was no relationship between both p53 and ER expression and myometrial and lymphovascular invasion (p>0.05). Relation between stromal and epithelial p53 expression and cervical invasion did not show any statistical significance.
D i s c u s s i o n
In the present study, epithelial p53 expression was found to be higher in the tumoral regions compared to non-tumoral regions but there was no statistically significant association with stromal p53 expression. The rate of p53 expression has been reported to range between 14-25% for low grade ECs and 2.5-69% for grade 3 ECs [9, 10]. Contrary to SC, overexpression of p53 is regarded as a late sign of carcinogenesis for grade 3 ECs [5]. Therefore, an increase in tumour grade in ECs is expected to be associated with a higher p53 expression [11]. Interestingly, our study did not show any significant relationship between tumour grade and p53 expression. This can be explained by the lower number of grade 3 cases (n:6) or the heterogeneity of p53 mutations in the tumours [5]. Halperin et al. previously described p53 as a marker of tumour aggressiveness and loss of differentiation in endometrial carcinoma [12]. The present study attempted to determine a possible relationship between cervical, myometrial and lymphovascular invasion and p53 stromal/epithelial expression. p53 stromal/epithelial expression was found to be higher in solely cervical invasion however this was not statistically significant (p>0.05). This result supports the idea that p53 expression can be a good marker of tumour aggressiveness in endometrial carcinoma. In our study, contrary to p53 expression, ER expression was rather found to be lower in the tumoral regions compared to non-tumoral regions.
However, this difference was only statistically significant for the stromal component (p < 0.05).ECs usually develop from endometrial hyperplasia which is known to be associated with the lack of progesterone or abundance of estrogen. Hyperplasia and early stage EC is generally linked with ER positivity however for late stage EC and advance disease state, ER becomes negative and this indicates worse prognosis [13-16]. In our study, stromal ER expression were found to be higher in hyperplastic versus hyperplasic and hyperplastic stroma with or without atypia compared EC.
Similar to findings from the Kriezman-Shefer et al. study which found a decrease in ER expression in tumour cells, our study showed a decrease in stromal ER expression, we believe this finding is associated with tumour invasiveness [14]. Just like them, we found different levels of ER expressions at both the tumour surface and deeper layers but unfortunately we did not find any statistical analysis. In addition to the lack of relationship between p53 expression and tumour grade, there was no relationship between ER expression and tumour grade. This contradicts the traditional knowledge of a negative correlation between ER expression and tumour grade reported in the current literature [17]. Especially, in the peritumoral endometrial stroma, a loss of ER activity can trigger paracrine signalling and glandular pathologies [17,18]. As a result, an early identification of stromal ER loss is necessary in understanding hyperplasia/EC sequence. However, similar to findings from Stanescy et al.’s study, our study showed that, tumour grade increases as ER stroma expression increases [11]. The lack of a significant relationship between tumour grade and ER expression can be explained by the low number of grade 3 tumours (n=6). A larger series including more grade 2 and 3 cases is required to help investigate stromal ER expression loss.
C o n c l u s i o n
As a conclusion, the present study found that, most EC cases have a higher p53 but lower ER expression. P53 expression can be used as an indicator of tumour aggressiveness. In addition to known factors that need to be considered when evaluating immunohistochemistry of endometrioid carcinomas and their precursor lesions, first glance importance point; should be given to epithelial expression of p53 and stromal expression of estrogen.
Figure 1: p53 and estrogen stromal and epithelial expression in the hyperplasia /carcinoma. (A) p53 was negative both of the stromal and epithelial cells in non-hyperplastic endometrium (200x) (B) ER was strongly positive both of the epithelial and stromal cells non-hyperplastic endometrium (100x) (C) p53 was negative in endometrial hyperplasia without atypia,too (100x) (D) ER was strongly positive in endometrial hyperplasia without atypia (100x) (E) p53 expression was positive in the epithelium in endometrial hyperplasia with atypia (200x) (F) ER expression was positive in the epithelium (more stronger than stromal cells) in endometrial hyperplasia with atypia (100x) (G) p53 was strongly positive, especially epithelial component in grade 3 endometrioid carcinoma (200x) (H) Loss of ER expression in the stromal cells in grade 3 endometrioid carcinoma but strongly positive in the epithelial cells (200x) (I) p53 expression was positive in the epithelium in low grade endometrioid carcinoma (200x) (J) Loss of ER expression especially in the stromal components in low grade endometrioid carcinoma (200x).
Article Info
Article Type
Research ArticlePublication history
Received: Tue 13, Feb 2018Accepted: Thu 22, Feb 2018
Published: Wed 07, Mar 2018
Copyright
© 2023 Nurhan Şahin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2018.10.001
Author Info
Ayse Nur Akatlı Cemil Colak Ebru Inci Coskun Ercan Yilmaz Nurhan Şahin Nusret Akpolat Umran Karabulut Dogan
Corresponding Author
Nurhan ŞahinBezmi Alem University Hospital Pathology Department, Istanbul, Turkey
Figures & Tables
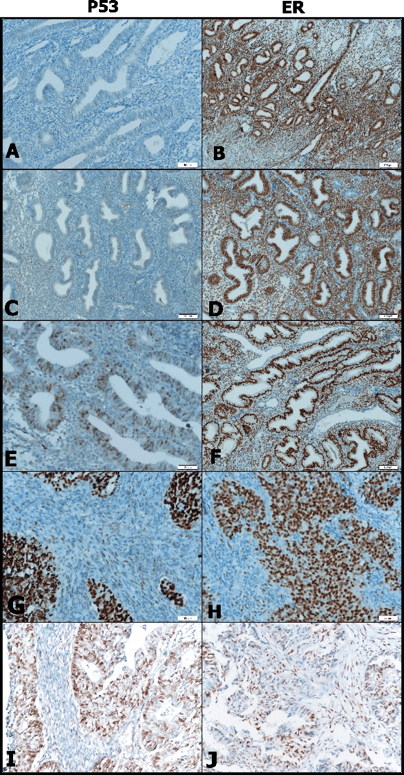
Fig. 1 - p53 and estrogen stromal and epithelial
expression in the hyperplasia /carcinoma. (A) p53 was negative both of the
stromal and epithelial cells in non-hyperplastic endometrium (200x) (B) ER was
strongly positive both of the epithelial and stromal cells non-hyperplastic
endometrium (100x) (C) p53 was negative in endometrial hyperplasia without
atypia,too (100x) (D) ER was strongly positive in endometrial hyperplasia
without atypia (100x) (E) p53 expression was positive in the epithelium in
endometrial hyperplasia with atypia (200x) (F) ER expression was positive in
the epithelium (more stronger than stromal cells) in endometrial hyperplasia
with atypia (100x) (G) p53 was strongly positive, especially epithelial
component in grade 3 endometrioid carcinoma (200x) (H) Loss of ER expression in
the stromal cells in grade 3 endometrioid carcinoma but strongly positive in
the epithelial cells (200x) (I) p53 expression was positive in the epithelium
in low grade endometrioid carcinoma (200x) (J) Loss of ER expression especially
in the stromal components in low grade endometrioid carcinoma (200x).
References
1- Yoshida M, Katsuda S, Maekawa A (2012) Involvement of Estrogen Receptor, Proliferating Cell Nuclear Antigen and p53 in Endometrial Adenocarcinoma Development in Donryu Rats. J Toxicol Pathol 25: 241-247.
2- Nguyen TT, Hachisuga T, Urabe R, Kurita T, Kagami S, et al. (2015) Significance of p53 expression in background endometrium in endometrial carcinoma. Virchows Arch 466: 695–702. [Crossref]
3- Geels YP, van der Putten LJ, van Tilborg AA, Lurkin I, Zwarthoff EC, et al. (2015) Immunohistochemical and genetic profiles of endometrioid endometrial carcinoma arising from atrophic endometrium. Gynaecol Oncol 137: 245–251. [Crossref]
4- Alvarez T, Miller E, Duska L, Oliva E (2012) Molecular Profile of Grade 3 Endometrioid Endometrial Carcinoma: Is it a type I or Type II Endometrial Carcinoma? Am J Surg Pathol 36: 753-761. [Crossref]
5- Feng YZ, Shiozawa T, Horiuchi A, Shih HC, Miyamoto T, et al. (2005) Intratumoral heterogeneous expression of p53 correlates with p53 mutation, Ki-67, and cyclin A expression in endometrioid-type endometrial adenocarcinomas. Virchows Arch 447: 816–822. [Crossref]
6- Atasoy P, Bozdogan O (2006) Molecular markers in endometrial hyperplasia. Gynecol Oncol 11: 61-67.
7- Bozdoğan O1, Atasoy P, Erekul S, Bozdoğan N, Bayram M (2002) Apoptosis related proteins and steroid hormone receptors in normal, hyperplasic and neoplastic endometrium. Int J Gynecol Pathol 21: 375-382. [Crossref]
8- Amalinei C, Cianga C, Balan R, Cianga P, Giusca S, et al. Immunohistochemical analysis of steroid receptors, proliferation markers,apoptosis related molecules, and gelatinases in non-neoplastic and neoplastic endometrium. Ann Anat 193: 43–55. [Crossref]
9- Silverberg SG (2014) Tumours of the uterin corpus; Epithelial tumours and related lesions. Lyon: IARC Press, (Kurman RJ, ed. WHO Classification of Tumours of the Female Reproductive Organs).
10-Koshiyama M, Ueta M (2002) Two kinds of endometrial neoplasia arising from different origins in the uterine corpus: comparison of p53 expression and sex steroid receptor status. Eur J Obstet Gynecol Reprod Biol 104: 167-170.
11- Stănescu AD, Nistor I, Potecă AG, Diţescu D, Comănescu M (2014) Prognostic biomarkers in endometrial adenocarcinoma. Rom J Morphol Embryol 55: 1339–1344. [Crossref]
12- Halperin R, Zehavi S, Habler L, Hadas E, Bukovsky I, et al. (2001) Comparative immunohistochemical study of endometrioid and serous papillary carcinoma of endometrium. Eur J Gynecol Oncol 22: 292-296. [Crossref]
13- Koshiyama M, Konishi I, Wang DP, Mandai M, Komatsu T (1993) et al. Immunohistochemical analysis of p53 protein over-expression in endometrial carcinoma: inverse correlation with sex steroid receptor status. Virchows Arch A Pathol Anat Histopathol 423: 265-71. [Crossref]
14- Kreizman-Shefer H, Pricop J, Goldman S, Elmalah I, Shalev E (2014) Distribution of estrogen and progesterone receptors isoforms in endometrial cancer. Diagn Pathol 9: 77. [Crossref]
15- Kounelis S, Kapranos N, Kouri E, Coppola D, Papadaki H, et al. (2000) Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod Pathol 13: 379-388. [Crossref]
16- Gul AE, Keser SH, Barısık O, Kandemir ON, Cakır C, et al. (2010) The relationship of cerb B2 expression with estrogen receptor and progesterone receptor and prognostic parameters in endometrial carcinomas. Diagn Pathol 5: 13. [Crossref]
17- Senol S, Sayar I, Ceyran AB, Ibiloglu I, Akalin I, et al. (2016) Stromal Clues in Endometrial Carcinoma: Loss of expression of β-Catenin, Epithelial-Mesenchymal Transition Regulators, and estrogen-Progesterone Receptor. Int J Gynecol Pathol 35: 238–248. [Crossref]
18- Smuc T, Lani'snik RT (2009) Aberrant pre-receptor regulation of estrogen and122-126. progesterone action in endometrial cancer. Mol Cell Endocrinol 301: 74-82.
