The Outcome of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Patients with Pancreatic Cystic Neoplasms: A Systematic Review
A B S T R A C T
Introduction: Pancreatic cancer is the sixth most common cause of death from cancer in the UK. Cystic pancreatic neoplasms are being recognized more with the increase in the use of the CT scan. EUS has been increasingly used to asses and identify lesions in the pancreas, however, it can’t differentiate between benign and malignant tumors alone. The role of EUS guided FNA cytology (EUS FNAC) is still controversial in the management of pancreatic cysts where neoplastic process is questioned.
Aim: This systematic review is aiming to explore the currently available evidence assessing the role of EUS guided FNA cytology (EUS FNAC) in the management of pancreatic cystic neoplasms.
Methods: A total of five studies with 597 patient EUS FNAC episodes were included in this systematic review.
Results: The sensitivity of the EUS FNAC in the papers was variable between 46.7% to 91.7% while the sensitivity of the test was 100% for all the papers except for 1 paper which was 82.1%. CEA level was assessed in 3 papers, however, the cut off level was different.
Conclusion: The high specificity of EUS FNAC qualify it as a useful adjunct to ascertain or exclude malignancy in the pancreatic cystic lesions. EUS FNAC cannot be used alone as a method of screening, given low sensitivity. Measuring CEA in the cyst fluid can be a good aide to increase the sensitivity and an identifiable cut off level should be proposed. Well-conducted and powered studies are needed to further explore the role of EUS FNAC in patients with pancreatic cystic neoplastic lesions.
Keywords
Endoscopic ultrasound scan, pancreatic cystic neoplasm, FNAC
Introduction and Background
Pancreatic cancer is the sixth most common cause of death from cancer in the UK and the fourth in the US. It constitutes around 2-3% of all cancers worldwide [1]. Pancreatic neoplasms can originate from the endocrine or the exocrine cells of the pancreas. Endocrine neoplasms can be functional or non-functional. Most of the endocrine tumors are malignant, but with better prognosis as compared to the exocrine tumors [2]. Pancreatic cystic lesions have been increasing over the last few years. They are a group of tumors with different characteristics, presentation and histology. Pancreatic cystic lesions are divided into neoplastic and non-neoplastic, 80% of these lesions are non-neoplastic [3]. All patients who are diagnosed with pancreatic cysts are being assessed for the possibility of malignancy, whether the cysts are symptomatic or not [4].
EUS has been increasingly used to asses and identifies lesions in the GI wall lumen, periluminal lymph nodes and intra-abdominal organs as the left lobe of the liver left kidney, pancreas, spleen and adrenal glands. However, EUS can't differentiate between benign and malignant tumors alone [5]. EUS guided FNAC is not without risks, these risks can be scope related, biopsy related, or sedation related [6]. These include GI perforations, aspiration, bacteremia, tumor dissemination, biliary peritonitis and cholangitis, acute pancreatitis, pancreatic leakage, haemorrhage, abdominal and thoracic pain, pneumothorax, pneumoperitoneum, and tumor seeding. The refined guidelines for the management of pancreatic mucinous cysts from the 2012 international consensus stated that small pancreatic cysts (< /= 3 cm) without high-risk stigmata for malignancy can be observed with repeated imaging only [4]. However, some recent studies showed that even small cysts and “Sendai negative” on imaging can contain up to 25% malignant cells inside [4]. Pancreatic cystic neoplasm attributes on EUS include cyst size of 3 cm, main pancreatic duct (PD) of 5-9 mm, thickened or enhancing cyst walls, a mural nodule, and an abrupt change in the calibre of the PD with distal pancreatic atrophy [7]. The role of EUS guided FNA cytology is still controversial in the management of pancreatic cysts not showing worrisome features on imaging4, however, several studies have been done to assess their use.
Two SRs were found, and they published in 2013 and 2015 aiming to assess the use of FNAC in the diagnosis of pancreatic cystic neoplasms. The first one in 2013 done by Thornton et al., assessing EUA guided FNAC and CEA levels in the diagnosis of pancreatic cystic neoplasms. They used as standard either surgical histology or follow up for more than 6 months. The result of the meta-analysis showed sensitivity and specificity for FNAC alone to be 54% and 93 % respectively and for the CEA to be 63% and 88% respectively [8]. The second SR was published in 2015 by Wang et al., to assess EUS guided FNAC in the pancreatic cystic neoplasms. The reference they used as their gold standard was either surgical resection or follow up for more than 6 months. For high-grade dysplasia and carcinoma, the sensitivity of the test was 94% and specificity was 51% [9]. Some of the papers included in this research seemed to have had more restrictions than the inclusion criteria Wang et al., described. For example, the paper published by Maire et al., in 2008 which only included intraductal papillary malignant neoplasms (IPMNs) and excluded all other cystic lesions [10]. The same was for Pais et al. paper which only included IPMNs [11].
There was another SR published in 2014 done by Suzuki et al., assessing the role of EUS FNAC in differentiating between the benign and malignant intraductal papillary malignant neoplasms (IPMN) but this only included papers showing IPMNs so it was not within the scope of this paper, however, it was used to check the references in the search process [12]. This piece of research is done to assess the published literature for the use of EUS FNAC to investigate the pancreatic cystic neoplasms and to assess its sensitivity and specificity in determining the malignancy in this category of pancreatic cysts.
Aim
This systematic review aims to explore the currently available evidence assessing the role of EUS guided FNA cytology in the management of pancreatic cystic lesions specially to differentiate malignant from benign pathology.
Material and Methods
This study is planned as a systematic review of the available literature. The last systematic review published was in 2015 [9]. Hence the decision to do a systematic review of the published literature in the last 7 years to further investigate the role of EUS guided FNAC.
I Study Selection for the Review
For this study the question was formulated as below: Is EUS guided FNAC sensitive and specific for screening for malignancy in patients with cystic pancreatic tumors as compared to the final diagnosis. A literature search was done in the search engines: Medline, CINAHL complete, AMED – The Allied and complementary Medicine Database and EMBASE. Medline and CINAHL were found to contain all the relevant research papers in that topic with no extra papers in the other search engines so the search was done through both of them. Another separate search was done through the Cochrane database to get systematic reviews on this topic and their references were searched to check for any papers that were not found in the search process. Keywords were chosen for the PICO structure as shown in (Table 1).
Table 1: Keywords for the search process.
|
Population (P) |
Intervention (I) |
Comparison (c) |
Outcome (O) |
|
Pancreas, Pancreatic. |
FNAC, FNAB, fine needle biopsy, fine-needle aspiration. Endoscopic ultrasound, |
Surgical resection, histopathology, surgical biopsy, definitive diagnosis. |
|
The comparison was made with the final diagnosis which was the surgical resection and histopathology if present or follows up with further imaging for at least 11 months for patients who didn't undergo surgery.
II Inclusion and Exclusion Criteria
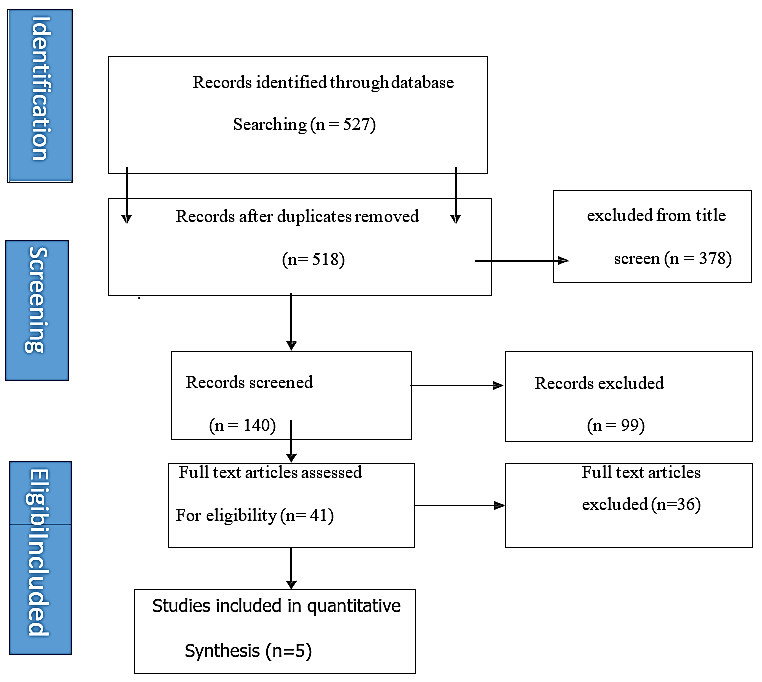
Inclusion and exclusion criteria for this systematic review were done according to the PICO/PIO format to avoid missing relevant studies (Figure 1).
Figure 1: Paper selection process.
1) Population
A) Inclusion Criteria:
i. Age: This study is aimed at adult patients, so it included papers with age groups older than 18.
ii. Gender: Both males and females were included in this study.
iii. FNAC for solid lesions were excluded.
iv. Papers only targeting neuroendocrine tumors were excluded.
B) Exclusion Criteria:
i. Age: Papers including only specific age groups e.g. Less than 35 only, were excluded from this study as this will affect the external validity of the study and make the study less representative of the general population.
2) Intervention
A) Inclusion Criteria: Papers with EUS guided FNAC for cystic pancreatic lesions were included.
B) Exclusion Criteria: i. Other methods of FNAC were excluded e.g. CT guided, or brush cytology.
3) Comparison
A) Inclusion Criteria: The final diagnosis was made based on surgical result and histopathology which is the gold standard, in cases where surgery was not indicated or didn't happen, the final diagnosis was based on surveillance for at least 11 months.
B) Exclusion Criteria: Papers with follow up less than 11 months were excluded.
4) Outcome
The primary outcome is the sensitivity and specificity. So, in papers with other parameters measured, papers were included when the statistical data allowed comparison between the EUS guided FNAC and the Final diagnosis.
5) Study Type
A) Inclusion Criteria:
i. Papers in the English language.
ii. Prospective or retrospective cohort and case-control studies to broaden the search outcomes.
iii. Papers published in the last 7 years.
B) Exclusion Criteria:
i. Other languages apart from English.
ii. Case series or case reports or studies with less than 20 cases.
Given that the 2015 guidelines recommend that patients with less than 2 high-risk feature to be followed up after a year with imaging and then on 2 yearly basis till year 5, and the 2017 revision of the Fukuoka guidelines the recommendation was the start of imaging after 6 months, then a year or two according to the size, it was felt that 6 months follow up is not enough to reach the endpoint and the need to increase the time of surgical follow up to be more than 11 months [13, 14]. The paper selection process was shown in the (Figure 1).
Data collection showed one prospective paper and four retrospective papers. For the selected papers, sensitivity and specificity were calculated if not already presented by the authors (Tables 2 & 3). There was one prospective observational study done by Wright et al. and published in 2018 titled: “Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions, a single-centre experience” which is aiming to compare the EUS guided FNAC according to the final diagnosis of follow up or the definitive pathology. The methods of getting the database, ethical approval, procedure technique, and statistical analysis were clearly explained, however, there were no power calculations to determine the required sample size that is required to provide statistical significance. There were no exclusion criteria for this study which improves its external validity. Of 120 cases in total undergoing EUS FNAC, 41 cases were relevant for this study. The total NOS score for the paper was 7 which indicates a good structure of the paper. For the results, the paper showed very good sensitivity and specificity of 91.7% and 100% respectively [15].
Table 2: Types of studies.
|
Paper |
Type of the study |
Comparison arm |
Length of Follow up |
|
Oguz1 |
Retrospective cohort |
Surgery and follow up |
2 years |
|
Zhan3 |
Retrospective cohort |
Surgery only |
N/A |
|
Phillip4 |
Retrospective cohort |
Surgery only |
N/A |
|
Woolf6 |
Retrospective cohort |
Surgery only |
N/A |
|
Wright7 |
Prospective cohort |
Surgery and follow up |
6-18 months |
Table 3: CEA levels.
|
Paper |
CEA measured or not |
CEA cut off |
|
Oguz1 |
Yes |
365, |
|
Zhan3 |
Yes |
692.8 |
|
Phillip4 |
No |
N/A |
|
Woolf6 |
No |
N/A |
|
Wright7 |
Yes |
192 |
In a study by Philip et al. and published in 2017 titled: “Evaluation of the 2015 AGA guidelines on pancreatic cystic neoplasms in a large surgically confirmed multicentre cohort”, which was a retrospective study aiming at evaluating the 2015 AGA guidelines if it is recommending surgical operations for malignant pancreatic cystic neoplasms appropriately or not. The data was collected from 2004 until 2014. This was a multicentric study done in 4 tertiary care centres in the United States. The exclusion criteria included lots of other cystic lesions which could affect the external validity. The criteria that they assessed the patients on was not the same, they analysed the studies on the Sendai guidelines until 2012 and on the Fukuoka guidelines after 2012. There was no calculation to the sample size which decreases the external validity. The total number of patients included in this study was 300 patients. NOS score was 6 for this paper indicating an adequate structure of the paper and good internal validity. The results of this paper showed a sensitivity of 83.3% and a specificity of 82.1% [16].
There were 3 papers published in 2013, the first paper by Oguz et al. titled: “Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions, a single- centre experience”, which is assessing the EUS FNAC as a differentiating factor between benign and malignant pancreatic cystic lesions. It included a good explanation of the methods and statistical analysis. The sample size was small consisting of 56 patients. Of this small sample, 7 patients were lost in the follow-up. The sensitivity and specificity were 62% and 100% respectively. The overall NOS score for this paper was 5, which is the lower limit that is accepted in this study and indicates the average structure of the paper [17].
The second paper in 2013 was done by Zhan et al. and titled: “Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography- guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms” which were assessing the value of CEA level in EUS FNAC in predicting whether the pancreatic mucinous cystic neoplasm is malignant or not. This paper studies a mere 20 patients where the number was low. The sensitivity of this test was 60% and specificity was 100%. NOS score was 5, indicating average study strength and adequate to be included in this review [18].
The last paper that was published in 2013, was done by Woolf et al. and was titled: “False-Negative Rate of Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Pancreatic Solid and Cystic Lesions With Matched Surgical Resections as the Gold Standard” and it aimed to assess the diagnostic errors and differences between EUS FNAC and surgical resection in determining pancreatic malignancy in solid and cystic lesions. Out of 766 patients analysed only 26 patients had cystic lesions which are a small sample. No sample size calculation was done to determine the required number of cases to be studied. The sensitivity and specificity were 46.7% and 100% respectively. The NOS score of this paper was 5 indicating adequate strength and suitable for inclusion in the review [19].
Results
The demographic data (Table 4) were documented in 4 of the 5 papers by Three of them showed more tendency in females rather than males while one showed more tendency in males [15-18]. Age in these Four papers were over 50 with a mean age of 53.8, 59, 62.6 and 63 respectively [15-18].
Table 4: Demographic data and sample size.
|
Study |
Sex M: F |
Age |
Patient numbers |
|
Oguz et al. |
19:37 |
53.8 |
56 |
|
Zhan et al. |
14:6 |
59 |
20 |
|
Woolfe et al. |
-------- |
-------- |
101 |
|
Phillip et al. |
187:113 |
62.6 |
300 |
|
Wright et al. |
52:59 |
63 |
120 |
The total sample size for the papers was in ascending chronological order 56 pts for Oguz et al., 20 patients for Zhan et al., 101 for Woolf et al., 300 patients for Phillip et al., and 120 for Wright et al. [15-19]. Out of them the number that was included in the final analysis for this systematic review after elimination of the lost to follow up patients and those who are not included were 49 patients, 20 patients, 26 patients,300 patients, and 41 patients respectively. The sensitivity of the EUS FNAC in the papers by ascending chronological order was 63%, 60%, 46.7%, 83.3%, and 91.7% [15-19]. The sensitivity of the test was 100% for all the papers except for Phillip et al., which was 82.1%.
CEA concentration in the cyst fluid was included in 3 papers as an additional marker [15, 17, 18]. Oguz et al. found that the CEA level at 365ng/ml gives a sensitivity of 100%and a specificity of 65% [17]. Zhan et al. found the CEA level to be 878.2 +/- 273.2 ng/mL in the malignant lesions and to be 514.6 +/- 227.9 ng/mL in premalignant lesions. They concluded a cut off level of more than 692.8 ng/ml with a sensitivity of 80% and specificity of 90% in predicting malignancy [18]. Wright et al. didn’t analyse the CEA in particular, however, he found its level to be unreliable as a single predictor [15].
Discussion
This systematic review sought to answer a question about the value of EUS FNAC in the evaluation of pancreatic cystic neoplasms. Pancreatic cystic lesions increase with age, 80% of these are pseudocysts which is more common in males. On the other hand, pancreatic cystic neoplasms are more common in females as compared to males [3].
Given the above data, it was expected that the female to male ratio to be high which was the case for three out of four papers, however, there was one paper that had a different ratio with more males than females. This different paper was done in a Chinese hospital in a military medical university. This might affect the population studied and raises the question of whether the external validity of this paper is adequate to generalize the results of this paper on the general population. All of the four papers which discussed the demographics showed the disease to be more common in people over 50 years old which is consistent with the disease under investigation [2].
The sample size that was included in the final analysis was small, all the papers apart from Phillip et al. included less than 50 patients with 2 of them included 20 and 26 patients. The only exception was Phillip et al., who included 300 patients in the study [16]. None of the papers did a power calculation, so the exact number of patients required to get significant results can’t be identified [16]. All the papers included in this research mentioned their ethical committee approval apart from Wright et al. paper. They didn't mention getting any ethical committee or board approval before starting this paper. The paper was done at Manchester Royal infirmary hospital in 2015 and was published in 2018. It can be assumed that they have got approval before starting the data collection, but it was not documented in the paper [15]. Three papers had their final decision of the pathology based on surgical histopathology which is the gold standard and was satisfying the inclusion criteria while two papers depended on both surgical histopathologies and follow up which was long enough to be fulfilling the inclusion criteria of this review [15-19]. In papers only depending on surgical pathology it gives better evidence due to avoidance of loss to follow up patients, and the delayed presentation. In this paper, it was planned to avoid this bias by getting a longer length of follow up.
The follow up in Oguz et al. paper was for at least 2 years, while in Wright et al., the follow up was between 6 and 18 months with no clear explanation for who had the 6 months follow up and the reason for not continuing longer period [15, 17]. It might be justified in case they had their final diagnosis either by surgery or further investigation, or they may have been lost in the follow-up and in this case these patients need to be adjusted for the final data analysis. The downside for the papers which only included surgical patients as the final diagnosis is that it might have only concentrated on the malignant cases who had surgery and missed other cases who were deemed not fit for surgery, also the benign cases wouldn't have been included and this might affect the final sensitivity and specificity calculations.
The sensitivity of the papers showed marked discrepancies in the values starting from 46.7% for Woolf et al., to 60% for Zhan et al., to 63 % for Oguz et al., then 83.3% for Phillip et al., and the highest was Wright et al., with 91.7% [15-19]. As for the specificity, all the papers showed the specificity of 100% except for Phillip et al., who showed the specificity of 82.1%. This variation in sensitivity and specificity can be related to lack of power calculations leading to errors. The adequate sample size is very important to make sure that any study yields an accurate estimation of sensitivity and specificity [20].
Three papers also included CEA concentration in the cyst fluid as an additional marker [14, 17, 18]. Oguz et al. assessed the CEA level at 365ng/ml and found the sensitivity to be 100%, however, the specificity fell to 65%. They found that when adding CEA and CA19.9 in blood and cyst fluid along with assessing the cyst size gave the best prediction in differentiating benign lesions from malignant with an accuracy of 88% [17]. Zhan et al. assessed several levels of CEA in the cyst fluid and found the level to be 878.2 +/- 273.2 ng/mL in the malignant lesions and to be 514.6 +/- 227.9 ng/mL in premalignant lesions. They concluded that a cut-off level of more than 692.8 ng/ml to be predictive of the presence of malignancy. This cut off showed the sensitivity of 80% and specificity of 90% [18].
In Wright et al. paper, although they didn’t analyse the CEA in particular, they mentioned some useful data. They had two false-negative cases from EUS guided FNAC in which the CEA level was over 5000 and the second over 7000 and turned out to be malignant. They also noticed two malignant cysts with low CEA levels of 15 and 25 ng/ml. From their paper, CEA levels appear to be helpful but unreliable as a single predictor [15]. On a systematic review and Meta-analysis carried by Thornton et al. in 2013 showed a collected sensitivity of 54% and specificity of 93% for EUS FNAC and when CEA was assessed it showed sensitivity and specificity of 63% and 88% respectively [8]. This is consistent with the results found by some of the papers in this study, however, the cut off value for the CEA is still not standardized so the CEA sensitivity and specificity can’t be compared together until there is a similar CEA level to be compared to.
Conclusion
We found in this systematic review, EUS FNAC holds high specificity very good and the same can be used to ascertain pancreatic neoplastic cystic neoplastic lesions. However, EUS FNA test can’t be used alone as a method of screening, as on critical appraisal, the sensitivity was low. These findings are in accordance with most of the previously published literature.
Measuring cyst fluid CEA level can be a good adjunct to the EUS FNAC to help increase the sensitivity of the test, however, this still needs further evaluation. There is a need for further studies on cyst fluid CEA levels and consensus on evidence-based CEA cut off level to improve EUS FNAC sensitivity. One such study should have a primary outcome of the CEA level and compare different levels with the sensitivities to be able to set a standardized CEA level for future references. This should assist in identifying the cystic malignant lesions with increased accuracy and used as a routine screening method for suspected neoplasms in patients with pancreatic cysts.
Ethical Approval
For this study, the required ethical approval was obtained from Teesside University board, also the ethical approval for each article was checked separately.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 30, Jul 2020Accepted: Mon 10, Aug 2020
Published: Fri 21, Aug 2020
Copyright
© 2023 Viswanath YKS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.08.23
Author Info
Ahmed Mehanna Viswanath YKS Talvinder Gill Anil Reddy Andrew Gilliam Venkatesh Shanmugam Sachin Shenoy Suvi Virupaksha Mayank Bhandari Gopinath Bussa
Corresponding Author
Viswanath YKSJames Cook University Hospital, Middlesbrough, Cleveland, UK
Figures & Tables
Table 1: Keywords for the search process.
|
Population (P) |
Intervention (I) |
Comparison (c) |
Outcome (O) |
|
Pancreas, Pancreatic. |
FNAC, FNAB, fine needle biopsy, fine-needle aspiration. Endoscopic ultrasound, |
Surgical resection, histopathology, surgical biopsy, definitive diagnosis. |
|
Table 2: Types of studies.
|
Paper |
Type of the study |
Comparison arm |
Length of Follow up |
|
Oguz1 |
Retrospective cohort |
Surgery and follow up |
2 years |
|
Zhan3 |
Retrospective cohort |
Surgery only |
N/A |
|
Phillip4 |
Retrospective cohort |
Surgery only |
N/A |
|
Woolf6 |
Retrospective cohort |
Surgery only |
N/A |
|
Wright7 |
Prospective cohort |
Surgery and follow up |
6-18 months |
Table 3: CEA levels.
|
Paper |
CEA measured or not |
CEA cut off |
|
Oguz1 |
Yes |
365, |
|
Zhan3 |
Yes |
692.8 |
|
Phillip4 |
No |
N/A |
|
Woolf6 |
No |
N/A |
|
Wright7 |
Yes |
192 |
Table 4: Demographic data and sample size.
|
Study |
Sex M: F |
Age |
Patient numbers |
|
Oguz et al. |
19:37 |
53.8 |
56 |
|
Zhan et al. |
14:6 |
59 |
20 |
|
Woolfe et al. |
-------- |
-------- |
101 |
|
Phillip et al. |
187:113 |
62.6 |
300 |
|
Wright et al. |
52:59 |
63 |
120 |
References
- Williams NS, Bulstrode CJ, O'Connell PR (2013) Bailey & Love's short practice of surgery 26th ed. Crc Press. 84: 1517.
- Brunicardi FC (2015) Schwartz’s principles of surgery.
- William R Brugge (2015) Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol 6: 375-388. [Crossref]
- Roseann I Wu, Won Jae Yoon, William R Brugge, Mari Mino-Kenudson, Martha B Pitman (2014) Endoscopic ultrasound‐ guided fine-needle aspiration (EUS‐FNA) contributes to a triple-negative test in preoperative screening of pancreatic cysts. Cancer Cytopathol 122: 412-419. [Crossref]
- Nirag C Jhala, Darshana N Jhala, David C Chhieng, Mohamad A Eloubeidi, Isam A Eltoum (2003) Endoscopic ultrasound-guided fine-needle aspiration: a cytopathologist’s perspective. Am J Clin Pathol 120: 351-367. [Crossref]
- Christian Jenssen, Maria Victoria Alvarez Sánchez, Bertrand Napoléon, Siegbert Faiss (2012) Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol 18: 4659-4676. [Crossref]
- Christopher W Teshima, Gurpal S Sandha (2014) Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol 20: 9976-9989. [Crossref]
- G D Thornton, M J W McPhail, S Nayagam, M J Hewitt, P Vlavianos et al. (2013) Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 13: 48-57. [Crossref]
- Qi Xian Wang, Jun Xiao, Matthew Orange, Hu Zhang, You Qing Zhu (2015) EUS- guided FNA for diagnosis of pancreatic cystic lesions: a meta-analysis. Cell Physiol Biochem 36: 1197-1209. [Crossref]
- Frédérique Maire, Hélène Voitot, Alain Aubert, Laurent Palazzo, Dermot O'Toole et al. (2008) Intraductal papillary mucinous neoplasms of the pancreas:performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol 103: 2871-2877. [Crossref]
- Shireen A Pais, Siriboon Attasaranya, Julia K Leblanc, Stuart Sherman, C Max Schmidt et al. (2007) Role of endoscopic ultrasound in the diagnosis of intraductal papillary mucinous neoplasms: correlation with surgical histopathology. Clin Gastroenterol Hepatol 5: 489-495. [Crossref]
- Rei Suzuki, Nirav Thosani, Srinadh Annangi, Sushovan Guha, Manoop S Bhutani (2014) Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: a systematic review and meta-analysis. Pancreatology 14: 380-384. [Crossref]
- James M Scheiman, Joo Ha Hwang, Paul Moayyedi (2015) American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148: 824-848. [Crossref]
- Masao Tanaka, Carlos Fernández Del Castillo, Terumi Kamisawa, Jin Young Jang, Philippe Levy et al. (2017) Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17: 738-753. [Crossref]
- P K Wright, D A Shelton, M R Holbrook, S A Thiryayi, N Narine et al. (2018) Outcomes of endoscopic ultrasound-guided pancreatic FNAC diagnosis for solid and cystic lesions at Manchester Royal Infirmary based upon the Papanicolaou Society of Cytopathology pancreaticobiliary terminology classification scheme. Cytopathology 29: 71-79. [Crossref]
- Phillip S Ge, V Raman Muthusamy, Srinivas Gaddam, Diana Marie Jaiyeola, Stephen Kim et al. (2017) Evaluation of the 2015 AGA guidelines on pancreatic cystic neoplasms in a large surgically confirmed multicenter cohort. Endosc Int Open 5: E201-E208. [Crossref]
- Oguz D, Öztaş E, Kalkan IH, Tayfur O, Cicek B et al. (2013) Accuracy of endoscopic ultrasound-guided fine-needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: A single-centre experience. J Dig Dis 14: 132-139.
- Xian Bao Zhan, Bin Wang, Feng Liu, Xiao Fei Ye, Zhen Dong Jin, Zhao Shen Li (2013) Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography‐ guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms. J Dig Dis 14: 191-195. [Crossref]
- Kirsten M W Woolf , Hua Liang, Zachary J Sletten, Donna K Russell, Thomas A Bonfiglio et al. (2013) False‐ negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution's experience. Cancer Cytopathol 121: 449-458. [Crossref]
- N M Buderer (1996) Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 3: 895-900. [Crossref]
