The Menstrual Cycle Related Hormone Variations and Breast Cancer Risk: A Novel Theory
A B S T R A C T
Background: The cumulative and excessive exposures to estrogen contributing to increased risk of breast cancer may be misleading.
Methods: We proposed a novel theory to highlight that exposure to unstable estrogen levels may play a critical role in determining breast cancer risk among females as the change of endocrine environment may pose a risky environment to breast tissues for carcinogenesis. Particularly, we considered the menstrual cycle related estrogen variation (MCREV) as the primary hazard to breast cancer among females.
Results: To describe MCREV, its intensity was defined as the difference between the highest and the lowest levels of estrogen within a menstrual cycle; its timing was defined as age at menarche and age at pregnancies (i.e. timing of cessation of exposure to this hazard); and its frequency and duration can both be defined as the total number of menstrual cycles, which is influenced by many factors including age of menarche, age of menopause, average length of one menstrual cycle, and durations and number of pregnancies and breastfeeding.
Conclusions: The proposed MCREV theory may identify women at high-risk of breast cancer at an earlier age. The development of breast cancer might be weakened if suitable techniques to reduce the MCREV become clinically available. This novel theory opens a new door in breast cancer prevention and management.
Keywords
Breast cancer, sex hormone, estrogen, menstrual cycle, variations of estrogen
Introduction
Estrogen plays a crucial role in development and regulation of the female body, influencing maturation of secondary sex characteristics and the menstrual cycle. Estrogen also performs other important endocrine functions, such as maintenance of bone mass, and regulation of insulin responsiveness [1]. There are four naturally occurring forms of estrogen in females, which are synthesized at different primary sites depending on life stage: estrone (E1) is synthesized in and secreted from the ovaries and adipose tissues, and one of the types of estrogens found in women after the menopause along with estradiol; estradiol (E2) is the most biologically active and predominant form of estrogen primarily synthesized in the ovaries and adipose tissues during reproductive years and is tied to the development of female cancers; estriol (E3) is primarily produced by the placenta and is virtually undetectable among women who are not pregnant; and estetrol (E4), which is only synthesized by the fetal liver during pregnancy. Other forms of metabolic sources of estrogen such as estrone sulfate (ES1), estradiol sulfate (ES2), estriol sulfate, and various other estrogen metabolites may be present as well.
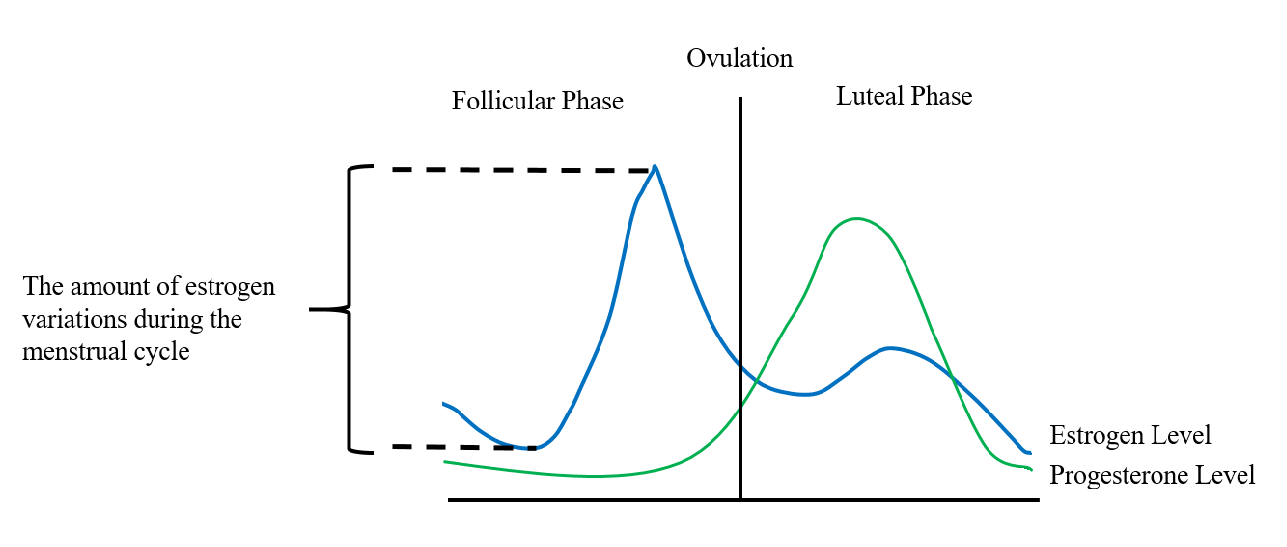
The menstrual cycle typically spans 25 to 30 days and consists of four phases: menstruation and follicular, ovulatory, and luteal phases. 10-12 hours after the peak in the luteal phase, ovulation occurs [2, 3]. The mean length is approximately 14.37 days for the follicular phase and 14.26 for the luteal phase [4]. Thus, setting the first day of menstruation as day 1, the cutoff threshold for the follicular phase and the luteal phase can be defined as the 14th day of the cycle [5]. Generally, E2 concentration is low at the beginning of the follicular phase, during which it steadily increases. A dramatic increase in E2 concentration followed by a rapid decline precedes ovulation, promoting surges in production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) which triggers release of the mature ovum. After ovulation, the remains of the dominant follicle in the ovary become a corpus luteum, which produce large amounts of progesterone to prepare the uterus for potential implantation of an embryo. If the ovum is not fertilized and implanted, the ovary will resorb the corpus luteum and levels of progesterone and estrogen will decrease (Figure 1) [2, 3].
Figure 1: Intensity of the hazard of breast cancer: the MCREV.
Breast cancer is one of the most common malignancies in women. An estimated number of 249,260 new cases of breast cancer occurred in the U.S. in 2016, of which 246,660 cases were women. There were approximately 40,450 deaths from breast cancer among women in 2016 [6]. Estrogen along with other sex hormones such as progesterone are critical regulators of breast development and functions including formation of ductal structures and elaboration of normal epithelial lobules [7, 8]. The link between estrogen and breast cancer has been recognized for over 100 years since bilateral oophorectomy was discovered to mitigate breast cancer in a young woman [9]. However, the exact mechanisms through which estrogen influences the risk of breast cancer remain unclear. Well established breast cancer risk factors such as early age at menarche, older age at first pregnancy, high bone density, obesity and late age at menopause tend to support the prevailing theory that cumulative exposure to estrogen is the principal factor of significance [10-15].
However, this cumulative estrogen exposure model can hardly explain these pregnancy-related protective effects as it is well known that estrogen levels steadily increase during pregnancy and reach a peak in the third trimester. Specifically, the levels of E2 in the first trimester range from 188-2497 pg/mL while the peak E2 levels during menstruation are only 100-400 pg/mL [16]. Thus, estrogen exposure levels during pregnancy are much higher than during non-pregnancy periods. Pregnancy-induced long-term cancer protection is conflicting with the cumulative estrogen exposure model. To better understand the link between estrogen and breast cancer risk, we propose a novel theory to describe changes in estrogen levels throughout a woman’s life course, i.e. the variation in estrogen levels under normal health conditions or other events (e.g. menarche, pregnancy, menopause), may play a crucial role in the development of breast cancer. In the following section, we described the characteristics of this proposed new theory, applied the theory to explain the well-established risk factors of breast cancer including menstrual patterns, reproductive history, and genetic factors, analyzed the potential biological mechanisms associated with the hazard, and discussed clinical and public health implications of this theory as well as future research directions in the field.
A New Theory: MCREV as a New Hazard for Understanding the Role of Estrogen in Breast Cancer Development
The frequency and amplitude of estrogen fluctuations throughout a woman’s life course may play a critical role in breast carcinogenesis. Major estrogen fluctuations can be observed during a woman’s menstrual cycles, after delivery of pregnancies, and in the transition to menopause, among which menstruation related cyclic estrogen fluctuations may be of the greatest importance because 1) a woman is exposed to this cyclic estrogen fluctuations from adolescence into adulthood during reproductive ages (fairly long time) and 2) the peak level of E2 (i.e. 360 pg/ml at pre-ovulatory) within a menstrual cycle could be approximately 10 times as much as the lowest level of E2 (i.e. 38 pg/ml at early follicular phase) (large disparity) [17]. We describe this cyclic estrogen fluctuations as menstrual cycle related estrogen variation (MCREV), and we propose MCREV as a new hazard for understanding the role of estrogen in the development of breast cancer.
MCREV can be defined by four parameters: intensity, timing, frequency, and duration. Specifically, intensity is calculated as the difference between the maximum and minimum serum estrogen concentrations observed during one menstrual cycle. We hypothesize that larger differences correspond to intervals of higher breast cancer risk (Figure 1). Timing is defined as the age at initial exposure to MCREV and age at temporarily pause of exposure to MCREV. We hypothesize that if the other three parameters are kept constant, a younger age at first exposure and an older age at temporarily pause of exposure correspond to a higher risk of breast cancer. Factors that affect the timing of exposure to this hazard include age of menarche and age of first full-term pregnancy (i.e. timing related to first pause of exposure to MCREV).
Additionally, frequency is determined by the average length of one menstrual cycle, i.e. the number of days of one menstrual cycle. We hypothesize that if the other three parameters are kept constant, higher frequency is related to increased risk of breast cancer. Finally, duration is defined as the number of years of menstruation. We hypothesize that if the other three parameters are kept constant, longer duration is associated with increased breast cancer risk. Factors influencing duration of MCREV include age at menarche, length of menstrual cycles, age at first pregnancy, number of pregnancies, and age at menopause. All factors which are related to the features of MCREV are summarized in (Table 1). In sum, we hypothesize that women with higher intensity of MCREV, younger age at first exposure to MCREV, higher frequency of exposure to MCREV during the life course, and longer cumulative duration of exposure to MCREV are at higher risk of breast cancer.
Table 1: Factors related to the features of the (MCREV).
|
Features of MCREV |
Measurements or related factors |
|
Intensity |
The absolute differences between the peak and the lowest estrogen levels within a menstrual cycle (See Figure 1) |
|
Timing |
Early menarche (earlier time of exposure to the hazard); Age of first full-term pregnancy (earlier time of temporary pause of exposure to the hazard) |
|
Frequency |
Menstrual cycle length (Shorter menstrual cycle length relates more frequent exposure to the MCREV) |
|
Duration |
Early menarche and late menopause; Number of pregnancies; Lactation (breastfeeding) |
MCREV as a New Hazard for Describing the Effects of Established Breast Cancer Risk Factors
I Menstrual Patterns and Breast Cancer
Menstrual cycle characteristics, such as early menarche, short cycle length, and late menopause have been consistently linked with breast cancer risk via the hypothesis that cumulative estrogen exposure is the common factor of consequence [18-20]. Alternatively, our proposed MCREV model emphasizes the influence of repeated stimulations of low and high estrogen levels on breast cancer risk.
i Early Menarche
Early menarche is the factor which affects the timing and duration of exposure to MCREV. Just like the earlier initiation of smoking has more pronounced adverse health effects, the early initiation of exposure to alternative low and high estrogen stimulations associated with menstrual cycles may be harmful to breast tissues. Epidemiological studies have consistently demonstrated that early menarche is related to increased risk of breast cancer [21-24]. Previous studies have regarded it as the factor contributing to the cumulative exposure to estrogen. In our new MCREV model, however, early menarche is related to the timing and duration of exposure to MCREV rather than estrogen itself.
ii Menstrual Cycle Length
Menstrual cycle length defines the average number of days of one menstrual cycle and is one of the critical determinants related to the cumulative number of menstrual cycles which a woman experience. Generally, longer length of menstrual cycle is associated with reduced cumulative number of menstrual cycles, which we think could be protective to breast cancer, while shorter length increases the number menstrual cycles during a fixed period of time and further increases breast cancer risk. Studies have showed that shorter cycle length is associated with increased risk of breast cancer; however, few studies have taken into account other characteristics of menstrual cycle when assessing the effect of cycle length on breast cancer risk [14, 25-28]. In our proposed theory, we provide a more comprehensive understanding of the role of menstrual cycle in breast cancer development by considering the characteristics of MCREV including intensity, timing, duration, and frequency together.
iii Age at Menopause
Age at menopause can be defined as the age of last menstrual bleeding [29]. Later age at menopause is associated with a higher number of menstrual cycles and greater cumulative exposure to MCREV, suggesting greater potential for MCREV to influence breast cancer risk.
iv Cumulative Number of Menstrual Cycles
Similar to the menstrual cycle length, the association between cumulative number of menstrual cycles and breast cancer risk is also widely studied. The larger lifetime cumulative number of menstrual cycles has been reported to be associated with a higher breast cancer risk in postmenopausal women [30, 31]. According to our proposed theory, the additional risks may be attributable to the exposure to more MCREVs other than the longer exposure to endogenous estrogens as reported elsewhere [30]. For a better understanding of the role of the cumulative exposure of menstrual cycles in breast cancer risk, it may be essential to consider the exposure intensity, in addition to the duration of exposure.
The above factors were reported to be associated with breast cancer and most of them have been investigated individually, yet no theory has been proposed to explain the underlying mechanisms as a whole. Our proposed MCREV theory provides an innovative frame to investigate these factors simultaneously by considering them to be related to the hazard of MCREV including the timing, frequency, duration, and intensity. Our new theory offers a comprehensive and systematic approach to uncover the role of each factor in the development of breast cancer and provides inspirations for future studies aiming to develop statistical models incorporating the MCREV parameters to predict breast cancer risk.
II Reproductive History and Breast Cancer
Pregnancy is the most significant modifiable breast cancer risk factor among women [32, 33]. Earlier age at first full-term pregnancy, more pregnancies, and longer breastfeeding duration have been associated with reduced breast cancer risk. Since estrogen level during pregnancies dramatically increases, and higher level of estrogen is associated with higher breast cancer risk, we are not able to use the “cumulative exposure to estrogen” theory (i.e. higher cumulative exposure to estrogen is associated with increased risk of breast cancer) to explain the protective effect of pregnancy-related events. On the other hand, according to our MCREV theory, pregnancy temporarily stops menstruation and thus eliminates exposure to the hazard of MCREV, which may eventually lead to reduced risk of breast cancer.
i Early Age of first Full-Term Birth
Early age at first full-term birth is associated with an overall reduced lifetime risk of breast cancer [33-35]. According to our proposed theory, early age at first full-term birth is an important actor which influences both timing and duration of exposure to MCREV (Table 1): the earlier the first full-term birth is given, the earlier the temporary pause of the exposure to the MCREV is; full-term pregnancy also shortens the duration of lifetime exposure to MCREV. Both of them lead to an earlier reduced exposure to MCREV and therefore a reduced risk of breast cancer.
ii Pregnancy-Induced Transient Breast Cancer Risk
Estrogen levels rise during pregnancy, increasing 30-fold before childbirth. After delivery, those levels drop significantly, highlighting a dramatic swing in estrogen levels from conception to delivery. Pregnancy has a dual effect on breast cancer risk as it transiently increases breast cancer risk shortly after childbirth but reduces the risk in later life [36-38]. The proposed MCREV model may help to explain the short-term increase and long-term decrease in breast cancer risk associated with childbirth.
iii Lactation and Breastfeeding
Longer durations of breastfeeding have been associated with reduced risk of breast cancer [34, 39]. The mechanism of reduced risk may be related to contraceptive effects of breastfeeding [40]. Further, infant suckling can prolong the duration of lactational amenorrhoea beyond 6 months, potentially reducing estrogen exposure.
III BRCA1/2 Mutations and Breast Cancer
Carriers of germline mutations in BRCA1/BRCA2 have increased risk of breast cancer. Studies describing changes in serum estrogen levels during a menstrual cycle among mutation carriers are scant. However, published evidence suggests that BRCA1/BRCA2 mutation carriers experience greater changes in endometrial thickness during the menstrual cycle compared to wild-type controls, which may indicate differences in estrogen exposure between mutation carriers and negative controls [41]. Moreover, BRCA1/BRCA2 mutation carriers have exhibited higher serum levels of E2 and progesterone during the luteal phase compared to wild-type controls, which further supports differences in estrogen exposure according to mutational status [41].
Potential Biological Mechanisms Underlying the Model
The human female breast is composed of adipose tissue, known as stroma, which supports a network of branching ducts called the parenchyma [42, 43]. Development of the ductal system commences in the fetus and wanes in early childhood until puberty. Under the influence of estrogen, extensive tissue modelling occurs at the onset of puberty. During this developmental stage, estrogen promotes ductal elongation and dichotomous branching of the parenchyma occur alongside increases in fibrous and fatty tissues comprising the stroma, resulting in formation of a mature complex mammary gland, while progesterone mainly triggers the formation of side branches from the mammary ducts during menstrual cycles and pregnancy [8, 44-47].
The carcinogenic impact of variable estrogen exposure intensities on breast tissue is largely unknown. However, these fluctuations result in highly proliferative cellular activity and extensive breast tissue modeling during each menstrual cycle, increasing the potential for carcinogenesis. The notable changes of breast during menstrual cycles including breast volume have been identified [48]. Breast symptoms including tenderness, swelling, pain, changes in texture associated with menstrual cycles, cyclical mastodynia for instance, have also been well noticed [49, 50]. Physical exam through ultrasound has shown significant changes of the elastic properties of breast tissues due to the fluctuation of sex hormones during menstrual cycles [51]. At the cellular level, studies have demonstrated that brief proliferative spurts of breast tissues occur through the menstrual cycle by a regulation of the sequential and combined action of estrogen and progesterone [52]. Particularly, the proliferative activities within the epithelial compartment of breast are high, which lead to an increase in complexity and size of breast lobules [45, 53, 54]. All evidence seems to point towards the extensive tissue remodeling during the menstrual cycle. The rise and fall of key sex hormones of estrogen and progesterone during the menstrual cycles significantly influence cell proliferation and deaths in breast tissues, which potentially increase the possibility of carcinogenesis in breast tissues. More experimental and clinical studies are needed to confirm these potential biological mechanisms.
The Implication of This New Theory
The proposed MCREV theory may facilitate identification of high-risk women at an earlier age. Moreover, the natural history of breast cancer development might be attenuated if suitable techniques to reduce the MCREV become clinically available. For example, the model may serve as a useful chemo-preventive measure.
Future Studies
Accurate quantification of MCREV is needed to evaluate its association with breast cancer risk. This involves evaluation of intra- and inter-patient measurements with attention to potential racial differences. Initial studies could include in vivo murine modeling of MCREV and in vitro cellular studies, with potential validation studies among breast cancer patients and matched controls, and long-term follow-up cohort studies among unaffected women. After confirming the validity of the model, we will need to figure out ways to determine individualized threshold of MCREV level that is associated with increased risk of breast cancer and identify risk factors that are closely related to the MCREV. Additional research regarding the protective effect of an early age at first full-term pregnancy and the association of postmenopausal estrogen concentrations with breast cancer risk may be addressed into the hypothesis. Furthermore, further research into the variation of progesterone level in the menstrual cycle and the use of oral contraceptives may provide additional insight into the relationship with estrogen level and the potential increase in the risk of breast cancer. These are critical steps before effective screening and/or intervention approaches can be developed.
Conclusion
The proposed MCREV theory provides a novel way of understanding the role of estrogen as a risk factor in the development of breast cancer by recognizing the importance of estrogen variations under normal health conditions including menstruation, pre- and post-pregnancy, and transition to menopause. MCREV may be the major determinant of breast cancer risk. The potential clinical and public health implications of this model are promising.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
TheoryPublication history
Received: Tue 17, Mar 2020Accepted: Wed 08, Apr 2020
Published: Tue 14, Apr 2020
Copyright
© 2023 Liang Xiaohui . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.01
Author Info
Liang Xiaohui Nicholas Taylor Vixey Fang Xiao Zhang
Corresponding Author
Liang XiaohuiSchool of Medicine, SUNY at Stony Brook, USA
Figures & Tables
Table 1: Factors related to the features of the (MCREV).
|
Features of MCREV |
Measurements or related factors |
|
Intensity |
The absolute differences between the peak and the lowest estrogen levels within a menstrual cycle (See Figure 1) |
|
Timing |
Early menarche (earlier time of exposure to the hazard); Age of first full-term pregnancy (earlier time of temporary pause of exposure to the hazard) |
|
Frequency |
Menstrual cycle length (Shorter menstrual cycle length relates more frequent exposure to the MCREV) |
|
Duration |
Early menarche and late menopause; Number of pregnancies; Lactation (breastfeeding) |
References
- Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P et al. (1992) Effects of low doses of transdermal 17 beta-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab 74: 1396-1400. [Crossref]
- Treloar AE, Boynton RE, Behn BG, Brown BW (1967) Variation of the human menstrual cycle through reproductive life. Int J Fertil 12: 77-126. [Crossref]
- Reed BG, Carr BR (2018) The Normal Menstrual Cycle and the Control of Ovulation. Endotext [Internet] 2000. [Crossref]
- Jolivet A, Gautray JP (1978) Clinical investigations of the menstrual cycle I. Dragram of the normal menstrual cycle. Fertil Steril 29: 40-42. [Crossref]
- Liu Y, Wang Y, Zhou L, Yin K, Yin W et al. (2015) Prognostic effect of the menstrual cycle on timing of surgery in premenopausal breast cancer patients. Am J Surg 210: 506-511. [Crossref]
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66: 7-30. [Crossref]
- Macias H, Hinck L (2012) Mammary gland development. Wiley Interdiscip Rev Dev Biol 1: 533-557. [Crossref]
- Javed A, Lteif A (2013) Development of the human breast. Semin Plast Surg 27: 5-12. [Crossref]
- Beatson GT (1896) On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Lancet 15: 153-179. [Crossref]
- Pike MC, Spicer DV, Dahmoush L, Press MF (1993) Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 15: 17-35. [Crossref]
- Russo J, Russo IH (2006) The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 102: 89-96. [Crossref]
- Subramani R, Lakshmanaswamy R (2017) Pregnancy and Breast Cancer. Prog Mol Biol Transl Sci 151: 81-111.
- Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR (1994) Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol 140: 1081-1090. [Crossref]
- Wallace RB, Sherman BM, Bean JA, Leeper JP, Treloar AE (1978) Menstrual cycle patterns and breast cancer risk factors. Cancer Res 38: 4021-4024. [Crossref]
- Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J et al. (2007) Critial assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endor Relat Cancer 14: 169-187. [Crossref]
- Abbassi Ghanavati M, Greer LG, Cunningham FG (2009) Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 114: 1326-1331. [Crossref]
- Baird DT, Fraser IS (1974) Blood production and ovarian secretion rates of estradiol-17 beta and estrone in women throughout the menstrual cycle. J Clin Endocrinol Metab 38: 1009-1017. [Crossref]
- Garland M, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ et al. (1998) Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol 147: 636-643. [Crossref]
- MacMahon B, Trichopoulos D, Brown J, Andersen AP, Aoki K et al. (1982) Age at menarche, probability of ovulation and breast cancer risk. Int J Cancer 29: 13-16. [Crossref]
- Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC (1985) Do regular ovulatory cycles increase breast cancer risk? Cancer 56: 1206-1208. [Crossref]
- Khalis M, Charbotel B, Chajes V, Rinaldi S, Moskal A et al. (2018) Menstrual and reproductive factors and risk of breast cancer: A case-control study in the Fez region, Morocco. PLoS One 13: e0191333. [Crossref]
- Brachetto Brian D, Moguilevsky L, Grinberg R, Itre H (1951) Age of menarche, menopause and duration of the menstrual life in women with breast cancer. Prensa Med Argent 38: 236-238.
- Staszewski J (1971) Age at menarche and breast cancer. J Natl Cancer Inst 47: 935-940. [Crossref]
- Collaborative Group on Hormonal Factors in Breast Cancer (2012) Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13: 1141-1151. [Crossref]
- Olsson H, Landin Olsson M, Gullberg B (1983) Retrospective assessment of menstrual cycle length in patients with breast cancer, in patients with benign breast disease, and in women without breast disease. J Natl Cancer Inst 70: 17-20. [Crossref]
- Kelsey JL, Gammon MD, John EM (1993) Reproductive factors and breast cancer. Epidemiol Rev 15: 36-47. [Crossref]
- Beiler JS, Zhu K, Hunter S, Payne Wilks K, Roland CL et al. (2003) A case-control study of menstrual factors in relation to breast cancer risk in African-American women. J Natl Med Assoc 95: 930-938. [Crossref]
- Terry KL, Willett WC, Rich Edwards JW, Hunter DJ, Michels KB (2005) Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 14: 1509-1513. [Crossref]
- Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK et al. (2013) Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J Natl Cancer Inst 105: 526-535. [Crossref]
- Chavez MacGregor M, Elias SG, Onland Moret NC, van der Schouw YT, Van Gils CH et al. (2005) Postmenopausal breast cancer risk and cumulative number of menstrual cycles. Cancer Epidemiol Biomarkers Prev 14: 799-804. [Crossref]
- Clavel Chapelon F, E3N Group (2002) Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control 13: 831-838. [Crossref]
- Meier Abt F, Bentires Alj M (2014) How pregnancy at early age protects against breast cancer. Trends Mol Med 20: 143-153. [Crossref]
- Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T et al. (2012) Reproductive history and breast cancer risk. Breast Cancer 19: 302-308. [Crossref]
- Ursin G, Bernstein L, Lord SJ, Karim R, Deapen D et al. (2005) Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer 93: 364-371. [Crossref]
- Ma H, Bernstein L, Pike MC, Ursin G (2006) Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res 8: R43. [Crossref]
- Bruzzi P, Negri E, La Vecchia C, Decarli A, Palli D et al. (1988) Short term increase in risk of breast cancer after full term pregnancy. BMJ 297: 1096-1098. [Crossref]
- Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M et al. (1994) Transient increase in the risk of breast cancer after giving birth. N Engl J Med 331: 5-9. [Crossref]
- Mathelin C, Annane K, Treisser A, Chenard MP, Tomasetto C et al. (2008) Pregnancy and post-partum breast cancer: a prospective study. Anticancer Res 28: 2447-2452. [Crossref]
- Collaborative Group on Hormonal Factors in Breast Cancer. (2002) Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 360: 187-195. [Crossref]
- Short RV (1993) Lactational infertility in family planning. Ann Med 25: 175-180. [Crossref]
- Widschwendter M, Rosenthal AN, Philpott S, Rizzuto I, Fraser L et al. (2013) The sex hormone system in carriers of BRCA1/2 mutations: a case-control study. Lancet Oncol 14: 1226-1232. [Crossref]
- Forsyth IA (1991) The mammary gland. Baillieres Clin Endocrinol Metab 5: 809-832. [Crossref]
- Tobon H, Salazar H (1974) Ultrastructure of the human mammary gland. I. Development of the fetal gland throughout gestation. J Clin Endocrinol Metab 39: 443-456. [Crossref]
- Silberstein GB, Van Horn K, Shyamala G, Daniel CW (1994) Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology 134: 84-90. [Crossref]
- Howard BA, Gusterson BA (2000) Human breast development. J Mammary Gland Biol Neoplasia 5: 119-137. [Crossref]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW et al. (1998) A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A 95: 5076-5081. [Crossref]
- Brisken C, O'Malley B (2010) Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178. [Crossref]
- Milligan D, Drife JO, Short RV (1975) Changes in breast volume during normal menstrual cycle and after oral contraceptives. Br Med J 4: 494-496. [Crossref]
- Preece PE, Mansel RE, Bolton PM, Hughes LM, Baum M et al. (1976) Clinical syndromes of mastalgia. Lancet 2: 670-673. [Crossref]
- Gateley CA, Mansel RE (1990) Management of cyclical breast pain. Br J Hosp Med 43: 330-332. [Crossref]
- Rzymski P, Skorzewska A, Opala T (2011) Changes in ultrasound shear wave elastography properties of normal breast during menstrual cycle. Clin Exp Obstet Gynecol 38: 137-142. [Crossref]
- Soderqvist G, Isaksson E, von Schoultz B, Carlstrom K, Tani E et al. (1997) Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am J Obstet Gynecol 176: 123-128. [Crossref]
- Anderson E, Clarke RB, Howell A (1998) Estrogen responsiveness and control of normal human breast proliferation. J Mammary Gland Biol Neoplasia 3: 23-35. [Crossref]
- Hilton HN, Clarke CL, Graham JD (2018) Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol 466: 2-14. [Crossref]
