The Effect of Dental Enamel Pre-Etching for Self-Etching Adhesives According to their Primer pH: An In Vitro Bond Strength, Etching Pattern and Adhesive Failure Evaluation
A B S T R A C T
Dental enamel pre-etching before the application of a self-etching adhesive (SEA) has different effects depending on the pH of the adhesive acidic monomer, being not always beneficial. This study aimed to evaluate the shear bond strength (SBS), etching pattern, and fracture type of different acidity SEAs, with and without previous phosphoric acid etching. One-hundred-sixty bovine incisors were subjected to SBS testing with the following adhesive systems: Adper-Prompt-L-Pop (APLP) (strong acidity), Futurabond NR (FB), AdheSE One F (AD) (intermediary acidity) and Clearfil SE (CSE) (mild acidity), with and without previous phosphoric acid etching. Results were evaluated applying both ANOVA and Tukey’s test. Besides, forty bovine incisors were used to assess etching patterns using scanning-electron-microscopy (SEM). Adhesive failure was evaluated, classifying bond failure as one of five types. SBS averages were (MPa): without pre-etching: APLP (20.61±11.84), CSE (17.29±10.16), FB (11.44±6.99), AD (7.88±4.85) and with pre-etching: APLP (16.17±9.68), CSE (25.96±11.75), FB (20.12±9.39), AD (14,28±9.42). Different enamel etching patterns were observed depending on each SEA’s pH and whether the surface was pre-etched. Most fracture failures were adhesive type. Less than 10 % were cohesive type. SBS improves when mild and intermediary strength SEAs are pre-etched. However, it decreases when strong SEAs are pre-etched.
Keywords
Adhesive failure, adhesive interface, enamel pre-etching, etching pattern, self-etching adhesive, shear bond strength
Introduction
In recent years, numerous new adhesive systems have been introduced onto the dental market with the purpose of simplifying the dentin/enamel bonding technique. These new adhesive systems have incorporated different types of acidic monomers into their compositions intending to eliminate the need to use phosphoric acid for etching the enamel or dentin surface; they are called ‘Self-Etching Adhesives’ (SEA). A considerable number of studies support the efficacy of these adhesives for treating dentin, beginning with Nakabayashi’s description of the hybrid layer and followed by other authors such as Van Meerbeeck et al., Sofan et al., or Zecin-Deren et al. [1-4]. However, it is not clear whether SEAs’ behaviour in the enamel substrate is satisfactory. Several studies have shown that SEAs produce weaker etching patterns on enamel surfaces [5]. Furthermore, when performing adhesion tests, they achieve lower shear bond strengths (SBS) than conventional adhesive systems that follow the total-etch technique and produce a higher rate of microporosity, leading to a marginal failure [6]. Therefore, it would appear that SEAs are not as appropriate a choice for enamel bonding as total-etch adhesives (TEA).
Treating only one substrate (enamel or dentin) is rare in restorative dentistry, something of a dilemma given that TEA will achieve a better outcome on one surface (enamel), while SEA will behave better on the other (dentin). Some authors proposed performing a mixed technique using a SEA on both substrates but pre-etching the enamel with phosphoric acid to improve adhesion on this surface [7, 8]. Self-etching adhesives are subdivided into three groups based upon their primer pH: strong adhesives (with a pH of 1 or below), intermediary adhesives (with a pH of approximately 1.5), and mild adhesives (with a pH of 2 or more) [9].
To date, not enough studies have evaluated the effect of pre-etching enamel with phosphoric acid before using SEA with primers of differing pH. The aims of this study were: 1) to determine whether pH levels of SEA primers exert an influence on shear bond strength (SBS); 2) to assess whether SBS is affected by applying total-etch before using SEA systems; 3) to compare etching patterns of SEAs of different pH using scanning electron microscopy (SEM) observation; 4) to assess changes to etching patterns of SEAs when the enamel is pre-etched; 5) to classify fracture types when debonding each SEA; 6) to determine if fracture type is influenced by pre-etching with phosphoric acid. The hypothesis under examination in the present study is that pre-etching the enamel when using a SEA could enhance or reduce SBS depending on the pH of the primer incorporated into the adhesive system.
Materials and Methods
I Teeth
Two hundred bovine upper central incisors visually intact and with no cracks on the enamel surface were used. The teeth were washed in distilled water to remove any traces of blood and then placed in a 0.1% thymol solution for a day. They were stored in distilled water in an incubator that maintained a constant temperature of 37ºC. The water was changed every 24 hours to avoid deterioration. All teeth were stored for less than one month before SBS testing was performed.
Of the sample described, one hundred sixty teeth were used for SBS testing. They were set in a 4 cm long silicon cylinder with an internal diameter of 3 cm, with their roots submerged in type IV plaster. The remaining forty teeth were used for SEM observations. Each of these teeth was cut at the level of the cementoenamel junction using a diamond bur (Komet FG 6076-016, Rock Hill, USA). The coronal portion was used in the study, discarding the root.
II Bonding Procedure
One hundred sixty teeth were divided into eight groups. Random distribution was made before the assignment, following the internet program (Link). All were previously cleaned and polished with a rubber cup and a polishing paste without fluoride (Détartrine®, Septodont, Saint-Maur, France). Buccal surfaces were roughened with a diamond bur (Komet FG 6847-012). A composite cylinder made with Spectrum® (Dentsply de Trey, Konstanz, Germany), shade A2, 2 mm in diameter and 2 mm long was bonded to the buccal surface of each tooth following the manufacturer’s instructions for each product. The compositions of the products tested are shown in (Table 1).
Table 1: Adhesives included in the evaluation with their pH, composition, the percentage in weight of each component and lot number.
|
Adhesive |
Composition |
% in Weight |
Lot No. |
|
Adper Prompt L- Pop |
Bisphenol A diglycidyl ether dimethacrylate (BISGMA) |
40-50 |
419614 |
|
pH= 0.8-1 |
Triethylene glycol methacrylate |
40-50 |
|
|
|
Tetrabutylammonium tetrafluoroborate |
1-10 |
|
|
|
Silane-treated silica |
1-7 |
|
|
|
|
|
|
|
|
PART A |
|
|
|
|
2-Propenoic acid, 2-Methylropenoic Acid, 2-Methyl, Phosphinicobis (Oxy-2,1-Ethandiyl) Ester |
40 |
|
|
|
Mono HEMA Phosphate |
15 - 30 |
|
|
|
Methacrylated Pyrophosphates |
15 - 30 |
|
|
|
Tris 2-(Methacryloyloxy) Ethyl Phosphate |
1 - 10 |
|
|
|
Ethylene Dimethacrylate |
< 2 |
|
|
|
Phosphoric Acid |
< 2 |
|
|
|
Bis (2,6-Dichlorobenzoyl) Butylphenyl Phosphine Oxide |
< 0.2 |
|
|
|
2-Hydroxyethyl Methacrylate |
< 0.2 |
|
|
|
4-Methoxyphenol |
< 0.2 |
|
|
|
Hydroquinone |
< 0.1 |
|
|
|
Bisphenol A Diglycidyl Ether Dimethacrylate (BISGMA) |
10-15 |
|
|
|
DL-Camphorquinone |
1-1.5 |
|
|
|
Ethyl 4-Dimethylaminobenzoate |
<2 |
|
|
|
|
|
|
|
|
PART B |
|
|
|
|
Water |
70 - 80 |
|
|
|
2-Hydroxyethyl Methacrylate |
20 - 30 |
|
|
|
2-Propenoic Acid, Polymer with Methylenebutanedioic acid |
< 2 |
|
|
|
|
|
|
|
Futurabond NR |
PRIMER |
|
|
|
pH= 1.4 |
Acidic adhesive monomer |
50-100 |
1034373 |
|
|
BIS-GMA |
5-10 |
|
|
|
2-Hydroxyethyl methacrylate |
5-10 |
|
|
|
|
|
|
|
|
BONDING |
|
|
|
|
ethanol |
50-100 |
|
|
|
initiator |
2,5-5 |
|
|
|
|
|
|
|
Clearfil SE Bond |
PRIMER |
|
|
|
pH= 2 |
Bisphenol A diglycidyl methacrylate |
25-45 |
00990A |
|
|
2-hydroxyethyl methacrylate |
20-40 |
|
|
|
|
|
|
|
|
BONDING |
|
|
|
|
2-hydroxyethyl methacrylate |
10-30 |
01557A |
|
|
Other ingredients: |
|
|
|
|
10-Methacryloyloxydecyl dihydrogen phosphate |
|
|
|
|
Hydrophilic aliphatic dimethacrylate |
|
|
|
|
DL-Camphorquinone |
|
|
|
|
Water |
|
|
|
|
Accelerators |
|
|
|
|
Dyes |
|
|
|
|
Others |
|
|
|
|
|
|
|
|
AdheSE One F |
Bis-methacrylamide dihydrogen phosphate |
5-25 |
NL9016 |
|
pH= 1.5 |
Isopropanol |
5-15 |
|
|
|
Potassium fluoride |
< 1 |
|
|
|
Acrylamidosulfonic acid |
1-10 |
|
|
|
Acrylamido amino acid |
5-20 |
|
|
|
Water |
|
|
|
|
Alcohol |
|
|
Group I: APLP (n = 20): a layer of Adper Prompt L-Pop adhesive (3M ESPE Dental Products, St. Paul, USA) was applied to the buccal surface for 15 seconds and light-cured for 10 seconds using a Demetron LC® lamp (Kerr Hawe, Orange, USA). Then, the composite cylinder was applied with a silicone key and light-cured for 40 seconds.
Group II: APLP-AE (n = 20): the buccal surface was pre-etched with 37% phosphoric acid gel (Total Etch, Ivoclar, Vivadent, Schaan, Liechtenstein) for 15 seconds. The enamel was then washed with water and dried with compressed oil-free air. A layer of APLP and the composite cylinder were applied following the same procedure as in group APLP.
Group III: FB (n = 20): the buccal surface was treated with Futurabond® NR (VOCO GmbH, Cuxhaven, Germany). A layer of a mixed liquid A and B was applied for 20 seconds and then light-cured for 10 seconds. The composite cylinder was bonded as in previous groups.
Group IV: FB-AE (n = 20): the enamel was pre-etched with phosphoric acid followed by the same protocol as in group FB.
Group V: CSE (n = 20): a layer of Clearfil SE® Bond (Kuraray, America Dental, New York, USA) was applied to the buccal surface. The primer was rubbed for 20 seconds and then light-cured for 10 seconds. The composite cylinder was bonded as in previous groups.
Group VI: CSE-AE (n = 20): the enamel was pre-etched with phosphoric acid before carrying out the same procedure as in group CSE.
Group VII: AD (n = 20): a layer of AdheSE One F (Ivoclar, Vivadent, Liechtenstein) was applied to the buccal surface. It was rubbed for 20 seconds and light-cured for 10 seconds. The composite cylinder was bonded as in previous groups.
Group VIII: AD-AE (n = 20): the enamel was pre-etched with phosphoric acid before carrying out the same procedure as in group AD.
III Storage of Test Specimens
When the bonding procedure had been performed, specimens were immersed in distilled water at a temperature of 37ºC for 15 days.
IV Bond Strength Test
SBS was measured with a universal testing machine (Autograph AGS-1KND, Shimadzu, Kyoto, Japan) with a 1 KN load cell connected to a metal rod with one end angled at 30 degrees. The crosshead speed was 1 mm/minute. The teeth were set at the base of the machine so that the sharp end of the rod incised into the composite cylinder, exerting a force parallel to the tooth surface in an inciso-apical direction. The force required to debone each composite cylinder was registered in Newtons (N) and converted into Megapascals (MPa) as a ratio of Newtons to surface area of the cylinder base (MPa=N/mm2).
V SEM Observation
Forty teeth were divided into eight groups (n=5). The same surface preparation protocol was followed for each group as in “Bonding Procedure,” but without bonding the composite cylinders. After this, all teeth were rinsed with acetone for 10 seconds to remove the self-etching primer resin [10]. Then, the teeth were cleaned in distilled water with ultrasonic agitation for 30 minutes and gently air-dried. They were then fixed to SEM stubs, sputter-coated with gold (Thermo VG Scientific, Bio-Rad. Polaron Division, East Grinstead, Great Britain) and examined under a Jeol-6100 (Tokyo, Japan) scanning electron microscope operating at 15 kV and with a working distance of 20 mm. Images at x250 and x800 were captured and stored digitally for each group.
VI Evaluation of Adhesive Failure
Adhesive failure type was determined using image analysis equipment (Sony dxc 151-ap video camera connected to an Olympus SZ11 microscope) and MIP4 Advanced software (Microm Image Processing Software, Digital Image Systems, Barcelona, Spain). Adhesive failure was classified into five different types:
Type I: Cohesive failure of the enamel.
Type II: Adhesive failure between bonding resin and enamel
Type III: Cohesive failure of the bonding resin
Type IV: Adhesive failure between bonding resin and composite
Type V: Cohesive failure of the composite.
To avoid intra-operator error in this evaluation, 32 teeth were evaluated twice with a time difference of 15 days by the same observer. Data were analysed using the T-test for two related samples and the Pearson correlation test (p < 0.05).
VII Statistical Analysis
SBS values for the eight bonding procedures were compared. Shapiro-Wilks’ normality test was applied to the shear bond strength data. As the data fulfilled the criteria for normality, significant differences were determined through variance analysis (ANOVA) and Tukey’s test for multiple comparisons (p < 0.05).
Results
I Shear Bond Strength Test
When the different adhesives were applied following the manufacturers’ instructions, that is without previous enamel acid etching, the higher shear bond strength was reached by the strong self-etch adhesive, Adper Prompt L Pop (20.61 ± 11.84) showing statistically higher shear bond strength values than intermediary strength adhesives: for FB (11.44 ± 6.99), p = 0.043 and AD (7.88 ± 4.85), p = 0.001 but did not show a statistically significant difference when compared to the mild adhesive: CSE (17.29 ± 10.16), p = 0.972. Furthermore, CSE also showed statistically higher shear bond strength values than one of the intermediary strong adhesives (AD: p = 0.05).
When the enamel surface was pre-etched with phosphoric acid, the mild adhesive (CSE) achieved statistically higher shear bond strength values (25.96 ± 11.75) than one of the intermediary strong adhesives, AD, (14.28 ± 9.42), p = 0.004 and the strong adhesive, APLP (16.17 ± 9.68), p = 0.035. No significance was found between FB (20.12 ± 9.39) and CSE, p = 0.054.
Tukey’s test was applied to data in order to compare each adhesive system’s SBS performance with and without (control group) pre-etching with phosphoric acid. The mild acidic adhesive (CSE) and intermediary strength adhesives (FB and AD) acquired significantly higher SBS values following pre-etching: FuturaBond (p = 0.09), Clearfil SE (p = 0.09) and AdheSE (p = 0.45). However, the more acidic adhesive (APLP) achieved lower SBS values following pre-etching, although without significant differences (p = 0.86) (Table 2).
Table 2: Shear Bond Strength expressed in MPa: means ± standard deviations. In brackets, the lowest and highest values reached in each group. The capital letters indicate statistically significant differences between the same adhesive system with and without previous acid etching. The presence of lowercase letters shows statistically significant differences between the different adhesives.
|
Adhesive System |
No Pre-etching |
Pre-etching |
|
Adper Prompt L-Pop |
20.61 ± 11.84 (3.18/47.11)ab |
16.17 ± 9.68 (4.93/37.72)e |
|
FuturaBond |
11.44 ± 6.99 (3.82/32.31)Aa |
20.12 ± 9.39 (4.93/35.65)A |
|
Clearfil SE |
17.29 ± 10.16 (5.41/42.02)Bc |
25.96 ± 11.75 (8.28/50.13)Bde |
|
AdheSE |
7.88 ± 4.85 (0.16/18.78)Cbc |
14,28 ± 9.42 (3.98/42.81)Cd |
II SEM Examinations of Enamel Surface
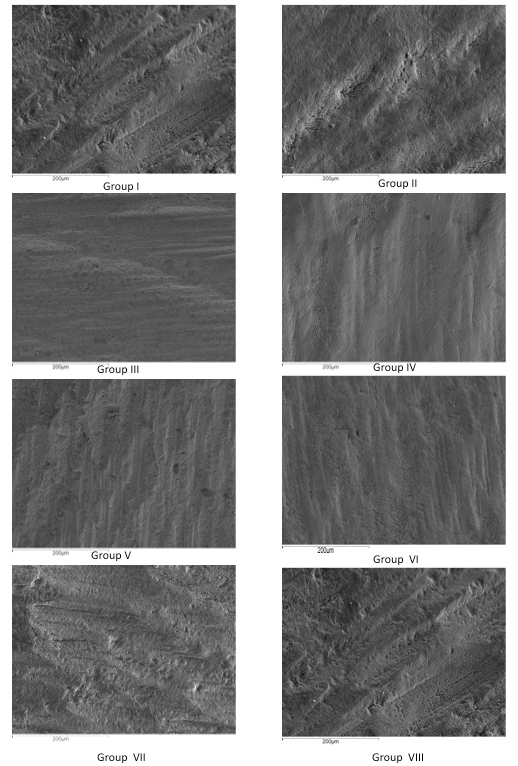
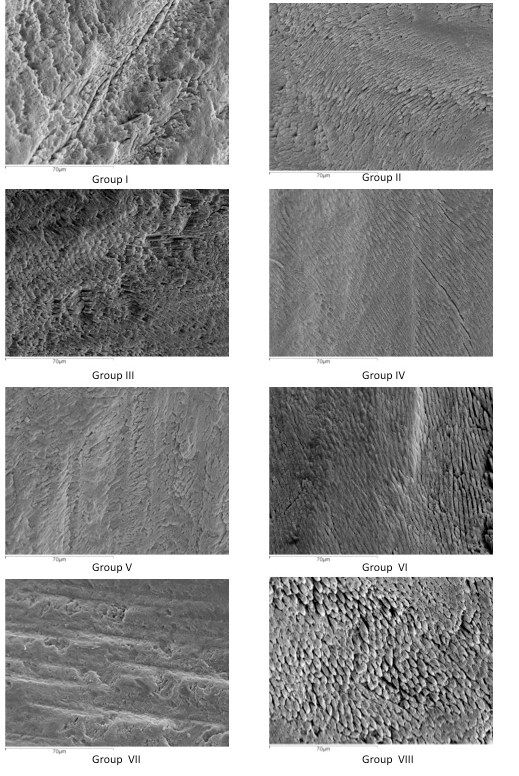
When scanning electron microscopy (SEM) micrographs were analyzed at low magnification (x250), a type V etching pattern was observed in the mild and intermediary strength SEAs without phosphoric acid pre-etching. There were isolated areas that showed slightly etched enamel prisms (type II). The most acidic adhesive (APLP) showed a type IV etching pattern with isolated regions of type II. With pre-etching, the mild and intermediary strength SEAs showed type II etching patterns, and the strong adhesive showed type III (Figure 1). At higher magnification (x800), when the different adhesives were pre-etched, mild acidic adhesives showed a more defined type II. However, the adhesive with the strongest acidity showed poorly defined enamel prisms (type III) (Figure 2).
III Failure Type Analysis
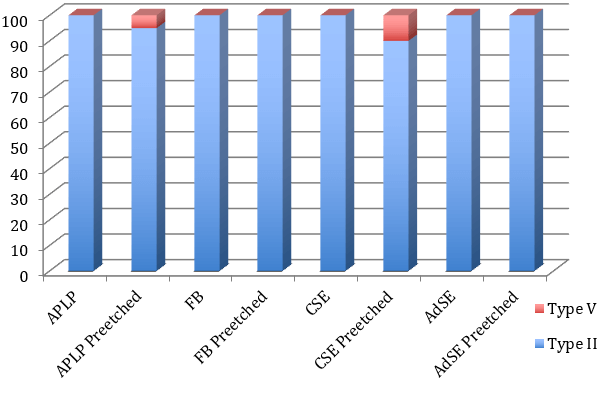
The microanalysis images of the fracture sites revealed that the predominant failure type was adhesive failure between bonding resin and enamel. However, APLP-AE showed a cohesive failure of the composite of 5%, and CSE-AE showed a 10% cohesive failure. All other debonded interfaces showed adhesive failure (Figure 3).
Figure 1: SEM images of each evaluated enamel surface at an x250 magnification.
Figure 2: SEM images of each evaluated enamel surface at an x800 magnification.
Figure 3: Graphic with the percentage distribution of adhesive failure in each of the groups evaluated.
Discussion
Bovine incisors were used in this study because of their microstructural similarity to human tooth enamel and also because of the easy availability of samples with intact vestibular enamel surface [11]. Several authors support the use of bovine incisors for research on dental bonding [12, 13]. In 1992 Watanabe and Nakabayashi introduced the concept of ‘acidic primers’ as a substitute for phosphoric acid for etching the dental structure to obtain a faster and easier enamel/dentin bond [14]. Later in 1993, the authors confirmed that enamel pre-etching could increase SEA shear bond strength values to levels similar to those achieved by ERA systems [15]. Since then, several authors have described good adhesion on dentine, while on enamel, better results are achieved using the traditional phosphoric acid technique [16, 17]. Torii et al. studied the idea of etching with 35% phosphoric acid for 15 seconds before applying acidic primer to enhance the enamel bond, and so achieve higher bond strengths on enamel, but this produces lower bond strengths on dentin; other authors have confirmed these results [18-20].
Authors such as Rotta et al., Ibrahim et al. argued that the pH of the acidic monomer embedded in the SEA might influence SBS results [9, 21]. Therefore, Ibrahim et al. found that intermediary strong SEA produced higher SBS values than mild and strong SEA [9]. However, their results do not agree with the present study, which found better SBS with the most acidic adhesive and that intermediary strength adhesives produced the lowest SBS values. Ibrahim et al. concluded that pre-etching improved SBS for all SEAs, particularly intermediary strength adhesives. The present study found that pre-etching did not improve SBS for the strong SEA, which was seen to decrease while the etching pattern worsened. Our results are similar to those obtained by Erickson et al., who did not find any improvement as a result of pre-etching Prompt L-Pop adhesive [7].
However, with mild and intermediary strong adhesives, our study agrees with Ibrahim et al.’s results, and it can be concluded that pre-etching can enhance SBS for both adhesive types. Pre-etching with phosphoric acid produced increases in shear bond strengths for the intermediary strength and mild adhesives (AD, FB, and CSE), a finding that coincides with Lürhs et al. [22]. Van Landuyt et al. also concluded that pre-etching improved the SBS for CSE and that pre-etching must only be performed on enamel, not on dentine [20].
Our results mainly agree with other authors such as Goracci et al., and Moura et al. with the exception of AD, which obtained the lowest SBS in our study, while Goracci et al. and Ibrahim et al. obtained higher values for this adhesive [6, 9, 23]. Ibrahim et al. and Rotta et al. found lower SBS values for APLP than other authors [9, 24]. Contrary to our results, Rotta et al. described an improvement in SBS for APLP when pre-etching [21]. These discrepancies might be due to the different methodologies applied. Carvalho et al. also found a decrease in SBS after pre-etching CSE, although this was not statistically significant [25].
There must be other factors apart from variations in pH that influence adhesive behaviour. To this end, Erickson et al. tested the concept that certain SEA chemical compositions may improve bond strength (for instance, the presence of monomers that interact with enamel hydroxyapatite [HAP] such as MDP [10-Methacryloyloxydecyl dihydrogen phosphate]) [7]. Fukegawa et al. and Yoshihara et al. proposed that mild SEA adhesion on the enamel must be partly due to lower chemical reactivity (nano layering) with HAP [24, 26]. In addition, the intense chemical interaction between MDP and HAP could be due to HAP surface dissolution induced by MDP absorption and subsequent calcium-MDP-salt deposition with solubility lower than HAP.
As for SEM observation at 250x, none of the groups obtained an etching pattern similar to that produced by phosphoric acid, as observed by Moura et al. [6]. Intermediary and mild adhesives showed a Galil and Wright’s type V etching patterns with some isolated areas of Silverstone’s type II etching pattern [27, 28]. Only the strong adhesive (APLP) showed a Galil and Wright’s type IV and some isolated areas of Silverstone’s type II etching pattern (Figures 1 & 2). This leads us back to the question posed by Moura et al. as to how these materials are capable of producing adhesion [6]. Following pre-etching, the intermediary strong and mild adhesives showed a Silverstone’s type II etching pattern and the strong adhesive a Silverstone’s type III, as enamel prism height decreases due to the effect of double etching with two strong acids (Figures 1 & 2). Most of the fracture failures were type II (adhesive failure between bonding and enamel), as observed by Lührs et al. [22]. Only a low percentage of fractures were type V (cohesive failure on composite) in some pre-etched groups.
In vitro studies have their limitations but are, nevertheless, necessary and useful making for initial evaluations of adhesive systems. Still, in vivo research must be carried out to confirm In vitro results. The clinical relevance of the present study lies in that when using a strong SEA, previous enamel etching with phosphoric acid is not recommended because it not only worsens SBS but also produces a worse etching pattern, a possible cause of lower SBS on enamel. However, when a mild or intermediary strong SEA is used, previous enamel etching with phosphoric acid is recommended to improve adhesion on enamel.
Conclusions
i. Among the different SEAs used following the manufacturer’s instructions, adhesives with lower pH obtained the highest SBS values.
ii. SBS of mild and intermediary SEAs increases following pre-etching while SBS of the strong SEA decreases following pre-etching.
iii. The etching pattern produced by mild and intermediary SEAs is similar to a type V. The strong SEA showed a type IV etching pattern with some isolated areas of type II.
iv. Mild and intermediary strong SEAs with pre-etching showed a type II etching pattern. After pre-etching, the strong SEA produced a type III etching pattern.
v. When no pre-etching with phosphoric acid was carried out, all fractures were due to an adhesive failure between bonding resin and enamel.
vi. All composite cohesive failures occurred following pre-etching with phosphoric acid.
vii. The system that showed the best behaviour on enamel was pre-etched Clearfil SE.
Author Contributions
Conceptualization: A.I.N.-S. and F.C.-G; methodology: A.I.N.-S; software: A.I.N.-S; validation: A.S.-P; formal analysis: A.S.-P; investigation: A.I.N.-S; resources: A.S.-P; data curation: A.I.N.-S; writing-original draft preparation: A.I.N.-S; writing-review and editing: F.C.-G and A.S.-P; visualization: F.C.-G; supervision: A.I.N.-S; project administration: A.I.N.-S; funding acquisition: F.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 06, Jun 2020Accepted: Tue 16, Jun 2020
Published: Tue 30, Jun 2020
Copyright
© 2023 Ana I. Nicolas-Silvente . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDOA.2020.01.05
Author Info
Ana I. Nicolas-Silvente Fernando Chiva-Garcia Arturo Sanchez-Perez
Corresponding Author
Ana I. Nicolas-SilventeDepartment of Restorative Dentistry, Medicine and Dentistry Faculty, Murcia University, Murcia, Spain
Figures & Tables
Table 1: Adhesives included in the evaluation with their pH, composition, the percentage in weight of each component and lot number.
|
Adhesive |
Composition |
% in Weight |
Lot No. |
|
Adper Prompt L- Pop |
Bisphenol A diglycidyl ether dimethacrylate (BISGMA) |
40-50 |
419614 |
|
pH= 0.8-1 |
Triethylene glycol methacrylate |
40-50 |
|
|
|
Tetrabutylammonium tetrafluoroborate |
1-10 |
|
|
|
Silane-treated silica |
1-7 |
|
|
|
|
|
|
|
|
PART A |
|
|
|
|
2-Propenoic acid, 2-Methylropenoic Acid, 2-Methyl, Phosphinicobis (Oxy-2,1-Ethandiyl) Ester |
40 |
|
|
|
Mono HEMA Phosphate |
15 - 30 |
|
|
|
Methacrylated Pyrophosphates |
15 - 30 |
|
|
|
Tris 2-(Methacryloyloxy) Ethyl Phosphate |
1 - 10 |
|
|
|
Ethylene Dimethacrylate |
< 2 |
|
|
|
Phosphoric Acid |
< 2 |
|
|
|
Bis (2,6-Dichlorobenzoyl) Butylphenyl Phosphine Oxide |
< 0.2 |
|
|
|
2-Hydroxyethyl Methacrylate |
< 0.2 |
|
|
|
4-Methoxyphenol |
< 0.2 |
|
|
|
Hydroquinone |
< 0.1 |
|
|
|
Bisphenol A Diglycidyl Ether Dimethacrylate (BISGMA) |
10-15 |
|
|
|
DL-Camphorquinone |
1-1.5 |
|
|
|
Ethyl 4-Dimethylaminobenzoate |
<2 |
|
|
|
|
|
|
|
|
PART B |
|
|
|
|
Water |
70 - 80 |
|
|
|
2-Hydroxyethyl Methacrylate |
20 - 30 |
|
|
|
2-Propenoic Acid, Polymer with Methylenebutanedioic acid |
< 2 |
|
|
|
|
|
|
|
Futurabond NR |
PRIMER |
|
|
|
pH= 1.4 |
Acidic adhesive monomer |
50-100 |
1034373 |
|
|
BIS-GMA |
5-10 |
|
|
|
2-Hydroxyethyl methacrylate |
5-10 |
|
|
|
|
|
|
|
|
BONDING |
|
|
|
|
ethanol |
50-100 |
|
|
|
initiator |
2,5-5 |
|
|
|
|
|
|
|
Clearfil SE Bond |
PRIMER |
|
|
|
pH= 2 |
Bisphenol A diglycidyl methacrylate |
25-45 |
00990A |
|
|
2-hydroxyethyl methacrylate |
20-40 |
|
|
|
|
|
|
|
|
BONDING |
|
|
|
|
2-hydroxyethyl methacrylate |
10-30 |
01557A |
|
|
Other ingredients: |
|
|
|
|
10-Methacryloyloxydecyl dihydrogen phosphate |
|
|
|
|
Hydrophilic aliphatic dimethacrylate |
|
|
|
|
DL-Camphorquinone |
|
|
|
|
Water |
|
|
|
|
Accelerators |
|
|
|
|
Dyes |
|
|
|
|
Others |
|
|
|
|
|
|
|
|
AdheSE One F |
Bis-methacrylamide dihydrogen phosphate |
5-25 |
NL9016 |
|
pH= 1.5 |
Isopropanol |
5-15 |
|
|
|
Potassium fluoride |
< 1 |
|
|
|
Acrylamidosulfonic acid |
1-10 |
|
|
|
Acrylamido amino acid |
5-20 |
|
|
|
Water |
|
|
|
|
Alcohol |
|
|
Table 2: Shear Bond Strength expressed in MPa: means ± standard deviations. In brackets, the lowest and highest values reached in each group. The capital letters indicate statistically significant differences between the same adhesive system with and without previous acid etching. The presence of lowercase letters shows statistically significant differences between the different adhesives.
|
Adhesive System |
No Pre-etching |
Pre-etching |
|
Adper Prompt L-Pop |
20.61 ± 11.84 (3.18/47.11)ab |
16.17 ± 9.68 (4.93/37.72)e |
|
FuturaBond |
11.44 ± 6.99 (3.82/32.31)Aa |
20.12 ± 9.39 (4.93/35.65)A |
|
Clearfil SE |
17.29 ± 10.16 (5.41/42.02)Bc |
25.96 ± 11.75 (8.28/50.13)Bde |
|
AdheSE |
7.88 ± 4.85 (0.16/18.78)Cbc |
14,28 ± 9.42 (3.98/42.81)Cd |
References
- Nakabayashi N, Kojima K, Masuhara E (1982) The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res 16: 265-273. [Crossref]
- Van Meerbeek B, Inokoshi S, Braem M, Lambrechts P, Vanherle G (1992) Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J Dent Res 71: 1530-1540. [Crossref]
- Sofan E, Sofan A, Palaia G, Tenore G, Romeo U et al. (2017) Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma) 8: 1-17. [Crossref]
- Zecin Deren A, Sokolowski J, Szczesio Wlodarczuk A, Piwonski I, Lukomska Szymanska M et al. (2019) Multi-layer application of self-etch and universal adhesives and the effect on dentin bond strength. Molecules 24: 345. [Crossref]
- Frankenberger R, Lohbauer U, Roggendorf MJ, Naumann M, Taschner MJ (2008) Selective enamel etching reconsidered: better than etch-and-rinse and self-etch? J Adhes Dent 10: 339-344. [Crossref]
- Moura SK, Pelizzaro A, Dal Bianco K, de Goes MF, Loguercio AD et al. (2006) Does the acidity of self-etching primers affect bond strength and surface morphology of enamel? J Adhes Dent 8: 75-83. [Crossref]
- Erickson RL, Barkmeier WW, Kimmes NS (2009) Bond strength of self-etch adhesives to pre-etched enamel. Dent Mater 25: 1187-1194. [Crossref]
- Watanabe T, Tsubota K, Takamizawa T, Kurokawa H, Rikuta A et al. (2008) Effect of prior acid etching on bonding durability of single-step adhesives. Oper Dent 33: 426-433. [Crossref]
- Ibrahim IM, Elkassas DW, Yousry MM (2010) Effect of EDTA and phosphoric Acid pretreatment on the bonding effectiveness of self-etch adhesives to ground enamel. Eur J Dent 4: 418-428. [Crossref]
- Kanemura N, Sano H, Tagami J (1999) Tensile bond strength to and SEM evaluation of ground and intact enamel surfaces. J Dent 27: 523-530. [Crossref]
- Oesterle LJ, Shellhart WC, Belanger GK (1998) The use of bovine enamel in bonding studies. Am J Orthod Dentofacial Orthop 113: 514-519. [Crossref]
- Perdigao J, Monteiro P, Gomes G (2009) In vitro enamel sealing of self-etch adhesives. Quintessence Int 40: 225-233. [Crossref]
- Soares CJ, Castro CG, Santos Filho PC, da Mota AS (2007) Effect of previous treatments on bond strength of two self-etching adhesive systems to dental substrate. J Adhes Dent 9: 291-296. [Crossref]
- Nakabayashi N, Watanabe A, Gendusa NJ (1992) Dentin adhesion of “modified” 4-META/MMA-TBB resin: function of HEMA. Dent Mater 8: 259-264. [Crossref]
- Watanabe I, Nakabayashi N (1993) Bonding durability of photocured phenyl-P in TEGDMA to smear layer-retained bovine dentin. Quintessence Int 24: 335-342. [Crossref]
- Cruz Gonzalez AC, Delgado Mejia E (2020) Experimental study of brackets adhesion with a novel enamel-protective material compared with conventional etching. Saudi Dent J 32: 36-42. [Crossref]
- Nicolas AI, Vicente A, Bravo LA (2010) The in vitro effect of repeated bonding on the shear bond strength with different enamel conditioning procedures. Eur J Orthod 32: 291-296. [Crossref]
- Torii Y, Itou K, Nishitani Y, Ishikawa K, Suzuki K (2002) Effect of phosphoric acid etching prior to self-etching primer application on adhesion of resin composite to enamel and dentin. Am J Dent 15: 305-308. [Crossref]
- Erhardt MC, Cavalcante LM, Pimenta LA (2004) Influence of phosphoric acid pretreatment on self-etching bond strengths. J Esthet Restor Dent 16: 33-40. [Crossref]
- Van Landuyt KL, Kanumilli P, De Munck J, Peumans M, Lambrechts P et al. (2006) Bond strength of a mild self-etch adhesive with and without prior acid-etching. J Dent 34: 77-85. [Crossref]
- Rotta M, Bresciani P, Moura SK, Grande RH, Hilgert LA et al. (2007) Effects of phosphoric acid pretreatment and substitution of bonding resin on bonding effectiveness of self-etching systems to enamel. J Adhes Dent 9: 537-545. [Crossref]
- Lührs AK, Guhr S, Schilke R, Borchers L, Geurtsen W et al. (2008) Shear bond strength of self-etch adhesives to enamel with additional phosphoric acid etching. Oper Dent 33: 155-162. [Crossref]
- Goracci C, Sadek FT, Monticelli F, Cardoso PE, Ferrari M (2004) Microtensile bond strength of self-etching adhesives to enamel and dentin. J Adhes Dent 6: 313-318. [Crossref]
- Yoshihara K, Yoshida Y, Hayakawa S, Nagaoka N, Irie M et al. (2011) Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater 7: 3187-3195. [Crossref]
- Carvalho AP, Turbino ML (2009) Can previous acid etching increase the bond strength of a self-etching primer adhesive to enamel? Braz Oral Res 23: 169-174. [Crossref]
- Fukegawa D, Hayakawa S, Yoshida Y, Suzuki K, Osaka A et al. (2006) Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res 85: 941-944. [Crossref]
- Galil KA, Wright GZ (1979) Acid etching patterns on buccal surfaces of permanent teeth. Pediatr Dent 1: 230-234. [Crossref]
- Silverstone LM, Saxton CA, Dogon IL, Fejerskov O (1975) Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res 9: 373-387. [Crossref]
