The Challenge of the Non-Compliant Patient: A Case of Caesarean Section Scar Ectopic Pregnancy
A B S T R A C T
Ectopic pregnancy is a quite common and life-threatening pregnancy. The most common site of ectopic implantation of a fertilized embryo is the fallopian tube. In extremely rare situations, the embryo can implant in other locations, which makes the diagnosis and management even more complex. Although close observation of a new pregnancy is key in the diagnosis and treatment of an ectopic pregnancy, there is still a major risk of life-threatening outcomes. This is a case report of a 31-year-old patient with a history of multiple pregnancies who presented to a community hospital in the spring of 2021 with an ectopic caesarean scar pregnancy. A diagnosis of ectopic pregnancy was on a timely basis, and surgical management was advised. Upon refusal of treatment and admission, pharmacological management was initiated, but patient compliance challenged the success of the therapy. Patient non-compliance to close follow-up resulted in a ruptured uterus. Emergency laparotomy with supracervical hysterectomy was performed as a life-saving procedure.
Keywords
Ectopic pregnancy, caesarean section scar ectopic, methotrexate, compliance
Introduction
Ectopic pregnancy is the implantation of a blastocyst in a location other than the endometrium. The incidence of ectopic pregnancies is difficult to estimate and varies across studies. A large-scale study of Medicaid recipients in 14 different states in the United States (US) reported a rate of 14.7 out of 1000 pregnancies from 2004 to 2008 [1]. Most commonly, ectopic pregnancies are located in the fallopian tubes. Cases with cervical, ovarian, or scar tissue implantation are all extremely rare situations. Of all ectopic pregnancies, implantation on a caesarean scar happens in 1 out of 2000 pregnancies [2]. This represents approximately 6% of ectopic pregnancies among patients who had a previous caesarean delivery [2]. Risk factors associated with this specific type of ectopic pregnancy are the same as tubal ectopic implantations, including previous ectopic pregnancies, history of pelvic inflammatory disease, assisted reproductive technology, and use of estrogen modulators [3].
The rate of caesarean scar pregnancies has been gradually increasing in the past decades in the US. Although an uncommon cause of ectopic pregnancy, the recent increase in the rate of caesarean deliveries is directly associated with this type of ectopic pregnancy [2]. A large systematic review shows that the risk of caesarean scar pregnancies is directly proportional to the number of caesarean deliveries in a given patient [4]. We report a case of a caesarean scar ectopic pregnancy initially treated with conservative management. However, due to the patient’s poor compliance with the treatment plan, an emergency surgical management with a supracervical hysterectomy was inevitable.
Case Report
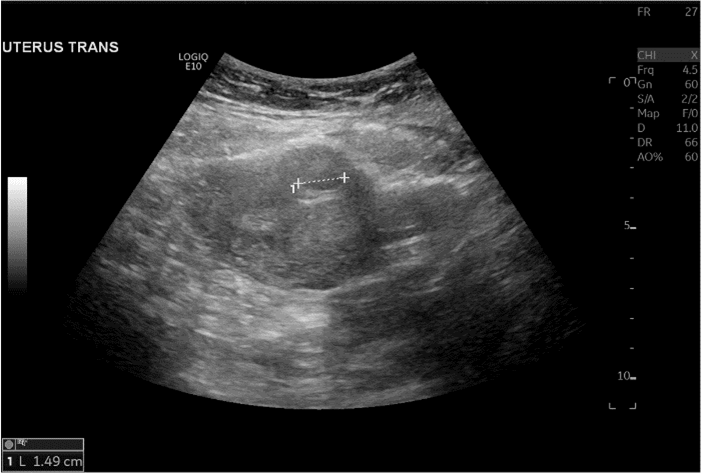
A 31-year-old G5P4004 female with a significant obstetric history of three vaginal deliveries and one caesarean section coupled with a medical history of diabetes mellitus, obesity, hypertension, bipolar disorder, and asthma was presented to Wyckoff Heights Medical Center’s Emergency Department complaining of lower abdominal pain. The pain was described as a constant, cramping pain rated 6 out of 10. She had been seen 8 days before in our emergency department with the complaint of abdominal pain and mild vaginal bleeding. Sonographic findings at that time revealed a 2.3 cm hypoechoic lesion within the posterior fundal aspect of the uterus consistent with a uterine fibroid and a 1.8 cm peripheral hypoechoic lesion in the anterior mid-uterus consistent with another uterine fibroid. No intrauterine pregnancy was visualized, and the b-HCG value was 11,301 IU/ml. The diagnosis of ectopic pregnancy was suspected, and the patient was advised admission for surgical management. The patient refused admission as well as surgery and signed out against medical advice. She followed up in the Women’s Health Clinic at Wyckoff Hospital two days later and her b-HCG value was documented as 15,534 IU/ml. She was sent for an additional sonogram which was consistent with an 8-week 3-day fetus with the implantation site on the previous caesarean section scar (Figures 1 & 2). The patient was counseled extensively on the need for admission to the hospital with surgical management. The patient still refused surgical treatment.
Figure 1: Transvaginal view of the gestational sac.
Figure 2: Transabdominal sonogram with a sagittal view of the gestational sac within the myometrium. Here the gestational sac is visualized within the right side of the endometrium, where the caliber is seen.
The patient was then counseled about the possible administration of methotrexate (MTX) and how it can be of use in ectopic pregnancies. It was explained to the patient that MTX could be given intramuscularly as well as at the site of the ectopic pregnancy. The patient agreed to the use of methotrexate. Under sonographic guidance by the Maternal-Fetal Medicine specialist, the gestational sac was localized transabdominal, and an 18-gauge amniocentesis needle was inserted in the gestational sac and 1cc of clear pink fluid was aspirated and sent for cytology. The solution with 50mg of methotrexate was instilled into the sac cavity and the patient tolerated the procedure well. She was then taken to the hospital’s emergency department for the intramuscular injection of 90mg of methotrexate based on her body surface area. The patient was still refusing admission and signed against medical advice. The patient understood that the ectopic pregnancy could rupture, resulting in maternal hemorrhage and death. The patient was given strict return precautions and told to follow up in 3 days for her day-4 b-HCG level following the methotrexate administration.
Unfortunately, the patient did not come for her day-4 b-HCG level. Eight days later, however, she came to the emergency department with severe abdominal pain. Upon clinical examination, she had stable vital signs but an acute abdomen. Her abdomen was tender to palpation, with rebound tenderness, and she had dark vaginal bleeding. The b-HCG value was 14,086 IU/ml. The patient was counseled on the likelihood of ruptured ectopic pregnancy and the immediate need for surgical management, to which she consented. The patient was taken to the operating room, and at laparotomy, a ruptured anterior uterine wall was noted with floating products of conception in the abdominal cavity. The decision was made to perform a supracervical hysterectomy. The total blood loss was approximately 200ml. The patient tolerated the procedure well and was discharged on postoperative day 1. The patient did not return to the clinic for follow-up. Multiple attempts were made to reach her and schedule an appointment. Pathology of the cyst aspiration came back as placenta accreta and gestational type endometrium. Diagnosis of the ruptured uterus and focal adenomyosis was made. Both fallopian tubes were histologically unremarkable.
Discussion
An abdominal scar following any invasive gynaecologic procedure such as a caesarean section or hysterectomy creates a favourable environment for ectopic blastocyst implantation. The early embryo can implant on the scar tissue itself in a microscopic wall defect of the healing uterus [5]. The mechanism of implantation in a caesarean scar is precipitated by a wedge defect in the uterus or a fistula within the scar [6]. The challenging clinical part of managing a caesarean scar ectopic pregnancy is that most patients are asymptomatic until critical presentation. As the pregnancy advances, the mother is at impending risk of uterine rupture resulting in critical hemoperitoneum from massive hemorrhage and hypovolemic shock [7]. Most patients will present in early pregnancy with the classic symptoms of abdominal pain and vaginal bleeding. Although unbearable pain can be a sign of impending uterine rupture, up to 40% of patients will present with mild to absolutely no symptoms until the scar is ruptured and the patient goes into critical condition [8]. A review of 112 cases of ectopic c-section pregnancies showed that the mean gestational age at presentation was 7.5 +/- 2.5 weeks [2]. This patient had a positive urine pregnancy test with a b-HCG of 16 at first.
Accurate location and dating of early pregnancy are crucial in preventing adverse events from a caesarean section scar ectopic pregnancy leading to maternal morbidity and mortality. Early prenatal care and ultrasound visualization of the pregnancy should be encouraged in women of reproductive age and not currently using any form of contraceptives. Appropriate localization of an ectopic pregnancy can be the key in directing treatment towards the least traumatic and invasive solution possible. Transvaginal ultrasound is highly accurate in assessing a new pregnancy location and is the main imaging modality in the first trimester [9]. Management decisions for each individualized patient are key to successful treatment. The treatment plan is dictated by the gestational age and size, location of implantation, the mother’s current situation and stability and her future desire for pregnancy. A comprehensive, empathic assessment of the patient’s compliance and understanding of the situation should be added in the decision-making of treatment. In hemodynamically unstable patients, emergent surgery is the main line of treatment. Only a few cases are being reported and treatment options are still an ongoing conversation in the medical community. For patients that wish to preserve their uterus and further fertility, surgical management by partial resection or aspiration tends to be preferred over methotrexate therapy. Both resection and aspiration are highly effective and avoid the risk of slow resorption and medical complications associated with methotrexate. In cases of resection and aspiration, the scar tissue can also be removed and prevent subsequent ectopic pregnancies. If the patient wants to pursue a conservative pharmacological therapy, intra-gestational injection of methotrexate for 1 mg/kg up to 50 mg is indicated [9]. Methotrexate therapy seems more efficient if administered intra-gestational than systemically [10].
A case report published in the “Journal of Medical Case Reports” urges the fact that early diagnosis of those types of ectopic pregnancy is the key to successful treatment [11]. It is mentioned that intrapartum methotrexate is preferred in a case of non-ruptured ectopic pregnancy to have the least invasive medical intervention possible. In the case of a ruptured embryo, a laparotomy intervention is often inevitable. Of note, the literature demonstrates that patients treated with local methotrexate did not need further management in 74% of cases [12]. This patient refused surgical management and received both intramuscular and intra-gestational dosage. She was not compliant with her follow-up appointment to evaluate the efficacy of the conservative and non-invasive methotrexate treatment. As her b-HCG kept rising, monitoring would have been crucial in management and decision-making.
A massive hemorrhage often necessitates transfusion of a large volume of blood products. Crossmatching before intervention is primordial. Blood transfusion also has major risks of infection and adverse reactions, renal and cardiovascular injury. The more the procedure is emergent, the more the risk of mortality is increased. This patient’s refusal of surgical management produced an unfavourable situation for appropriate management. Empathy and comprehension of a patient’s needs and reality are at the center of patient care in situations where compliance is an issue. A follow-up study of 18 cases advanced that intra-gestational methotrexate could be considered as first-line therapy for termination of caesarean scar ectopic pregnancy. Of note, all patients were compliant with treatment. In this study, 61% of patients did not need further management and 20% only needed an additional dose of methotrexate for successful termination of unviable pregnancy [12]. Had our patient complied with the follow-up plan outlined to her at the time of her discharge from the hospital after the methotrexate administration, it would have been evident that she required an additional dose of the drug or even a timely surgical procedure and thereby avoiding the risks of significant preoperative blood loss or death.
Article Info
Article Type
Case ReportPublication history
Received: Wed 04, Aug 2021Accepted: Fri 20, Aug 2021
Published: Fri 03, Sep 2021
Copyright
© 2023 Jessica Audet. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2021.02.01
Author Info
Brittany Noel Robles Nicolle M Arroyo Lluberas Jessica Audet Daniel Faustin Ralph Ruggiero
Corresponding Author
Jessica AudetXavier University School of Medicine, Oranjestad, Aruba
Figures & Tables

References
1. Stulberg DB, Cain
LR, Dahlquist I, Lauderdale DS (2014) Ectopic pregnancy rates and racial
disparities in the Medicaid population, 2004-2008. Fertil Steril 102:
1671-1676. [Crossref]
2. Rotas MA, Haberman
S, Levgur M (2006) Cesarean scar ectopic pregnancies: etiology, diagnosis, and
management. Obstet Gynecol 107: 1373-1381. [Crossref]
3. Li C, Zhao WH, Zhu
Q, Cao SJ, Ping H et al. (2015) Risk factors for ectopic pregnancy: a
multi-center case-control study. BMC Pregnancy Childbirth 15: 187. [Crossref]
4. Fylstra DL (2002)
Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv 57: 537-543. [Crossref]
5. Ash A, Smith A,
Maxwell D (2007) Caesarean scar pregnancy. BJOG 114: 253-263. [Crossref]
6. Marchiolé P, Gorlero
F, de Caro G, Podestà M, Valenzano M (2004) Intramural pregnancy embedded in a
previous Cesarean section scar treated conservatively. Ultrasound Obstet
Gynecol 23: 307-309. [Crossref]
7. Vial Y, Petignat P,
Hohlfeld P (2000) Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol
16: 592-593. [Crossref]
8. Ginath S, Malinger
G, Golan A, Shahmurov M, Glezerman M (2000) Successful laparoscopic treatment
of a ruptured primary abdominal pregnancy. Fertil Steril 74: 601-602. [Crossref]
9. Society for
Maternal Fetal Medicine (SMFM). Electronic address: pubs@smfm.org, Miller R,
Timor Tritsch IE, Gyamfi Bannerman C (2020) Society for Maternal-Fetal Medicine
(SMFM) Consult Series #49: Cesarean scar pregnancy. Am J Obstet Gynecol 222:
B2-B14. [Crossref]
10. Birch Petersen K,
Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H (2016) Cesarean scar pregnancy:
a systematic review of treatment studies. Fertil Steril 105: 958-967. [Crossref]
11. Jo EJ, Cha HH, Seong WJ (2019) Delayed diagnosis of a cesarean scar pregnancy: a case report. J Med Case Rep 13: 53. [Crossref]
12. Cheung VYT (2015) Local Methotrexate Injection as the First-line Treatment for Cesarean Scar Pregnancy: Review of the Literature. J Minim Invasive Gynecol 22: 753-758. [Crossref]
