Targeting Cancer Stem Cells and Metastasis with Epigenetic Modulation and Anti-HER2 Therapy: Phase I/II Trial of Vorinostat in Combination with Lapatinib
A B S T R A C T
Purpose: Considerable preclinical and clinical data indicate that only a small subset of tumor cells has long-term proliferating capacity. These cells are termed cancer stem cells (CSCs). Failure to eradicate CSCs is hypothesized to be a cause of cancer recurrence after potentially curative therapies. Therefore, approaches that target CSCs have the potential to improve outcomes. We evaluated the combination of vorinostat and lapatinib to target CSCs and metastasis.
Experimental Design: We conducted preclinical studies and a phase I/II clinical trial to determine the effects of vorinostat and lapatinib to CSCs.
Results: Our preclinical studies demonstrated that vorinostat and lapatinib further reduced CSCs compared to either single agent. Reduction in self-renewal proteins, mammospheres, epithelial-mesenchymal transition (EMT) markers, and cell migration was also observed. Based on these findings, the combination was evaluated in the phase I trial to which a total of 12 patients were enrolled. Dose-limiting toxicity was not observed in phase I, and the recommended phase II dose was vorinostat 400 mg 4 days on 3 days off and lapatinib 1,250 mg daily. In HER2-positive breast cancer patients, the clinical benefit rate was observed in 43% of subjects. Interestingly, patients who remained on vorinostat and lapatinib did not develop any new site of metastasis.
Conclusion: The combination of vorinostat and lapatinib is safe and active in HER2-positive breast cancer. Further studies are needed to evaluate this strategy to target CSCs and metastasis.
Keywords
Vorinostat, lapatinib, HDAC inhibitor, cancer stem cell
Statement of Significance
Vorinostat in combination with lapatinib significantly reduces CSCs self-renewal capacity and prevents metastasis. The combination of vorinostat and lapatinib is safe and active in HER2-positive breast cancer.
Introduction
A wealth of data supports the hypothesis that cancer cells are heterogeneous in their proliferative capacity, and only a distinct subset of tumor cells contributes to long-term tumor growth. These cancer cells have been termed tumor-initiating cells or cancer stem cells (CSCs). Unlike a stochastic model, which implies that all tumor cells have an equal capacity to proliferate, the CSC hypothesis suggests that tumor cells are hierarchically organized similar to normal tissues, and only certain tumor cells can sustain long-term tumor growth and differentiate into various lineages [1]. Furthermore, CSCs also share several biological properties with normal tissue-specific stem cells, including self-renewal capacity, plasticity, and ability to migrate.
Epithelial-mesenchymal transition (EMT) is the cellular process that leads to the acquisition of mesenchymal properties in epithelial cells. This process involves the loss of cell adhesion and acquisition of migratory capability. EMT is the physiological process that occurs during fetal development, including implantation, embryogenesis, and organ development, as well as during tissue regeneration later in life [2]. Multiple studies have demonstrated the association between EMT and CSC characteristics in several cancer types [1, 3]. In breast cancer, overexpression of Twist and Snail, which are transcription factors involved in EMT, has been shown to increase in the presumptive CSC population, measured by CD44hiCD24low and mammosphere formation. Conversely, cells expressing CSC markers CD44hiCD24low also exhibit EMT phenotypes [3]. Our group and others have demonstrated that human epidermal growth factor receptor 2 (HER2) is involved in self-renewal and expansion of CSC in breast cancer, particularly in the luminal subtype [4-6]. Furthermore, inhibition of HER2 using either trastuzumab or lapatinib has been shown to reduce the CSC population both in preclinical and clinical studies [4, 5, 7, 8].
Histone deacetylase inhibitors (HDACi) are a class of drugs that induce epigenetic changes. In our previous study, we demonstrated that HDACi could inhibit cell migration by reversing EMT. In addition, HDACi also downregulated HER2 and reduced the CSC population [9]. In a phase II trial of single agent vorinostat in metastatic breast cancer, although there was no objective response observed, 4 out of 14 patients had prolonged stable disease (SD) up to 14 months with this drug [10]. Based upon these findings, we initiated a clinical trial to further explore the combination of vorinostat in combination with lapatinib to target CSC and reduce metastasis by inhibiting cell migration and reversing EMT. The goal of our study was to investigate the safety and efficacy of this combination in phase I/II clinical trial.
Materials and Methods
I Materials
Vorinostat was provided by Merck (Kenilworth, NJ). Lapatinib was purchased from Selleck Chemicals (Houston, TX) for preclinical studies and was provided by GlaxoSmithKline (Philadelphia, PA) for the clinical trial. Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12), ATCC-grade RPMI-1640 Medium, trypsin/EDTA, penicillin/streptomycin (P/S), dPBS, Near IR viability dye, and vimentin antibody were purchased from Invitrogen (Waltham, MA). Fetal Bovine Serum (FBS) was purchased from Atlanta Biologicals (Flowery Branch, GA). HER2, Keratin 8/18, Bmi1, β-catenin, and β-actin were purchased from Cell Signaling Technology (Danvers, MA). Twist1 antibody was purchased from Abcam (Cambridge, MA). ALDEFLUOR kit, MammoCult media, and MammoCult supplements were purchased from Stem Cell Technologies (Vancouver, BC). CD24-FITC, CD44-APC, and CD49f-PE were purchased from BDPharmingen (San Jose, CA). Protease (Complete) and phosphatase (PhosSTOP) inhibitors were purchased from Roche (Basel, Switzerland). Pierce BCA Protein Assay Kit, SuperSignal West Pico Chemiluminescent Substrate, and Restore Western Blot stripping buffer were purchased from Thermo Scientific (Waltham, MA). All other western blotting materials were purchased from Bio-Rad (Hercules, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
II Cell Culture
Cells used for experiments included SUM149 kindly provided by Dr. Stuart Martin (University of Maryland, Baltimore, MD), BT474, and HCC1954 (both obtained from American Type Culture Collection). Cell lines were authenticated by the University of Maryland, Baltimore using short tandem repeat profiling in May 2015. SUM149 were routinely maintained in DMEM/F12, supplemented with 10% FBS, 1% P/S, 10µg/mL insulin, and 5µg/mL hydrocortisone. BT474 were routinely maintained in DMEM, supplemented with 5% FBS and 1% P/S. HCC1954 were routinely maintained in ATCC grade RPMI 1640, supplemented with 10% FBS and 1% P/S. All cell lines were maintained at 37°C in 5%CO2 and were passaged weekly. For cell treatment, each cell line was seeded and allowed to grow to 70% confluency. Cells were then treated for 72h with either vehicle (0.2% DMSO) or treatment (vorinostat and lapatinib were prepared as a 10-3 mol/L stock in DMSO).
III Western Blotting
Protein (25µg) was resolved by SDS-PAGE at 150V for 1h onto 4-15% Criterion Midi gels and transferred to polyvinylidene difluoride membrane. The resulting membranes were blocked and probed with designated primary and secondary antibodies. Blots were developed using SuperSignal West Pico Chemiluminescent Substrate. Blots were stripped with Restore Western Blot Stripping Buffer for 30 minutes at room temperature before incubation with another primary antibody. Densitometry was performed using ImageJ software, and densitometric values were normalized to loading control.
IV Real-Time Cell Migration with xCELLigence
Real-time measurement of cell migration was performed using xCELLigence RTCA DP (ACEA Biosciences, San Diego, CA). Cells were pretreated for 48h with designated treatments and then switched to a serum-free medium in the presence of treatments for an additional 24h. Cells were collected by trypsinization, neutralized with 1x soybean trypsin inhibitor, and counted. Then, 50,000 cells were seeded per well of a 16-well microelectronic sensored, 2-chamber trans-well plates containing respective drug in serum-free medium supplemented with 0.1% BSA. Media containing 5% FBS/0.1% BSA was added to the bottom wells. Migration was measured from the interaction of cells with electrodes on the bottom surface of the top chamber. This interaction is represented as a change in cell index (CI), an arbitrary unit derived from the relative change in electrical impedance across microelectronic sensor arrays. The electrical impedance was captured every 3 min for an experimental duration of 40 hours. The rate of migration is expressed as the CI or change in electrical impedance at each time point. Values are expressed as the mean ± standard error of the mean (SEM) of duplicate wells.
V Cell Surface Staining
Treated cells were collected by trypsinization and counted. Then, 1x106 cells were resuspended in 1mL dPBS and incubated for 30min with 1µL/mL Near IR cell viability dye on ice. Cells were spun and washed once with dPBS. Cells were resuspended in 100µL dPBS and 5µL CD24-FITC, 5µL CD44-APC, and 5µL CD49f-PE. Cells were incubated for 15 minutes at 37°C, spun, and washed with dPBS. Cells were fixed with 3.7% formaldehyde for 10 minutes at RT, washed, and stored in 1%BSA/dPBS at 4°C until acquisition. For ALDEFLUOR assay, similar preparation was employed, and the staining was performed according to the package insert. Cells were acquired by FACSCanto flow cytometer with appropriate compensation controls. Data were analysed using FlowJo software.
VI Mammosphere Assay
Treated cells were collected by trypsinization and counted. Then, 2,000 viable cells were seeded in Mammocult media (Mammocult media + supplements, 4µg/mL heparin and 0.48µg/mL hydrocortisone) in UltraLow attachment plates and allowed to propagate for 3 weeks at 37°C in 5%CO2. Mammospheres were counted manually by two independent operators, and the average was taken. Spheres with a colony count of at least 50 cells were considered mammospheres. For secondary passage, mammospheres were collected and centrifuged for 5 minutes at 400xg. The cell pellet was triturated using 1mL trypsin/EDTA and up/down pipetting using a P1000 pipette tip. Cells were resuspended in HBSS containing 2% FBS and centrifuged. Seeding was repeated as described above.
VII Clinical Trial Design
We initiated an open-label, single-arm, single institution, phase I/II trial at the University of Maryland Greenebaum Cancer Center. The primary objective of the phase I portion was to assess the safety and tolerability of the combination of vorinostat and lapatinib and determine the recommended phase II dose of this combination. The primary endpoint of phase II was clinical benefit rate (CBR), defined as the proportion of patients whose best overall response, according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1), was either complete response (CR), partial response (PR), or SD ≥ 6 months (REF) [11]. Secondary endpoints included progression-free survival and correlative studies. Enrollment in the phase II study was halted after 6 patients were enrolled due to the lack of funding. The protocol was reviewed by the institutional review board, and all patients provided written informed consent.
VIII Patient Selection
Phase I Cohort
Female or male patients with advanced solid tumor malignancies refractory to curative or standard palliative therapies who had a life expectancy greater than 3 months were eligible.
Phase II Cohort
Female or male patients with histologically confirmed HER2-positive (immunohistochemistry 3+ or fluorescence in situ hybridization ≥ 2.2) adenocarcinoma of the breast whose disease progressed after anthracycline, taxane, and trastuzumab were eligible. Measurable disease by RECIST criteria was required, but patients with bone only metastases were also eligible provided that there was a positive bone scan confirmed by MRI or PET/CT scan within 30 days prior to study entry. Prior trastuzumab and/or lapatinib therapy was allowed, but trastuzumab and/or lapatinib had to be discontinued at least 3 weeks prior to enrollment.
Patients in both cohorts were required to have Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, age ≥ 18 years old, and adequate organ function. Patients with prior exposure to HDACi (valproic acid ≥ 30 days was allowed); ≥ 5 prior lines of chemotherapies for stage IV breast cancer; significant cardiac disease; significant gastrointestinal disorder, particularly diarrhea; active central nervous system (CNS) metastasis (treated and stable CNS disease was allowed); known HIV or hepatitis B or C; active hepatic or biliary disease; uncontrolled intercurrent illness; and other malignancy within 3 years were excluded from this trial.
IX Treatment Procedures
Treatment cycles were 21 days. Lapatinib was given continuously at a fixed dose of 1,250 mg oral daily. In the phase I part of the study, vorinostat was administered in sequentially rising dose levels according to the standard 3+3 dose-escalation design to establish the maximum tolerated dose. There were 2 escalated dose levels starting with dose level (DL) 1. The dose of vorinostat in DL1 was 300 mg oral daily for 4 consecutive days, followed by 3 days off, and DL2 was 400 mg for 4 days on and 3 days off. The dose level was escalated if ≤ 1 of 6 patients experienced a dose-limiting toxicity (DLT) during cycle 1. If DLT was observed in DL1, vorinostat dose was to be de-escalated to DL-1 at 200 mg for 4 days on and 3 days off.
Treatment-related adverse events (AEs) were graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTCAE) version 4.0. DLT was defined as an absolute neutrophil count (ANC) < 500/mm3 lasting > 7 days; failure of ANC to recover to ≥ 1,000/mm3 within 14 days; platelets < 25,000/mm3, despite transfusion lasting > 7 days; failure of platelets to recover to ≥ 50,000/mm3 within 14 days; anemia with hemoglobin ≤ 7.9 g/dL, despite transfusion lasting > 7 days; grade ≥ 3 nonhematologic AEs (except for nausea/vomiting, if manageable); grade ≥ 3 diarrhea lasting > 2 days, despite being treated with optimal medical therapy; or grade ≥ 3 fatigue lasting > 7 consecutive days. Due to potential cardiac toxicity of lapatinib and QTc prolongation concern, patients enrolled in this trial also had an echocardiogram performed every 3 months and an electrocardiogram to evaluate QTc prior to starting treatment, 24-72 hours after the first dose of vorinostat, and at week 4 after treatment.
X Statistical Analysis
For preclinical studies, all experiments were performed using three replicates and were replicated at least twice. All data were expressed as mean ± SEM. P values were calculated by one-way ANOVA and Tukey post hoc analyses, using GraphPad Prism 5; p<0.05 was considered significant. For the clinical trial, descriptive statistics summarizing the number and percentage of patients with AEs according to the NCI CTCAE v4.0 were generated for all patients. No formal statistical analysis was performed on safety and efficacy data. The safety analysis population consisted of all patients who received at least 1 dose of study treatment.
Results
I Preclinical Studies
i Combination of Vorinostat and Lapatinib Decreases CSCs
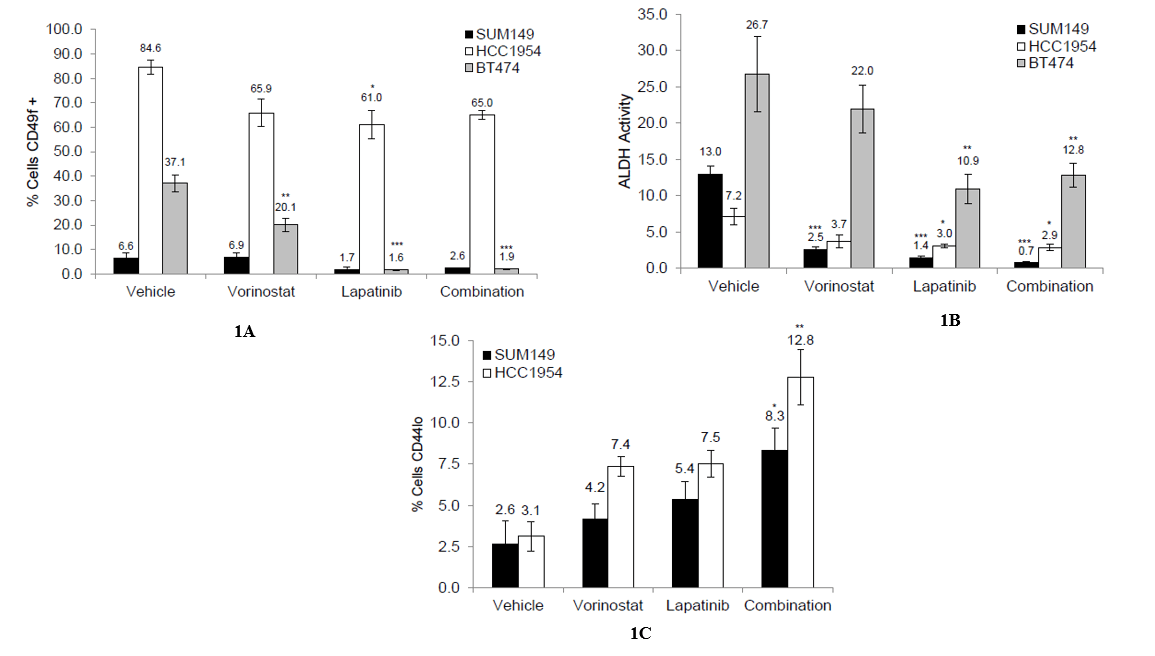
We first examined the effect of the combination on the expression of CD49f, a mammary epithelial marker associated with multipotency and stemness, in various HER2-positive breast cancer cell lines, including SUM149, BT474, and HCC1954 [12]. All three cell lines expressed CD49f, but at different levels; the highest expression was observed in the HCC1954 cell line, and the lowest was observed in the SUM149 cells (Figure 1A, Supplementary Figures 4-6). When treated with the single agent lapatinib or the combination, the expression of CD49f was reduced, though this reduction was only significant in the BT474 cell line (p<0.001 vs. vehicle control, Figure 1A). Next, we examined the effect of the combination on the activity of aldehyde dehydrogenase (ALDH), a marker of normal and malignant mammary stem cells [13]. Similar to CD49f expression, the cell lines differed in levels of ALDH activity, but all three showed a significant decrease in activity following treatment with the single agent lapatinib and the combination (p<0.001 [SUM149], p<0.05 [HCC1954], p<0.01 [BT474] vs. vehicle-treated control, Figure 1B, Supplementary Figures 4-6).
Figure 1:
Lastly, we examined the effect of the combination on the expression of cell surface markers CD24 and CD44. High expression of CD44 and low expression of CD24 have been associated with tumorigenicity, stemness, and metastasis in breast cancer [14, 15]. None of the treatments affected the expression of CD24 on the cell surface, but all three treatments were able to decrease the expression of CD44 on the cell surface, with treatment with the combination significantly decreasing expression in both the SUM149 and HCC1954 cell lines (p<0.05 [SUM149], p<0.01 [HCC1954] vs. vehicle-treated control, Figure 1C, Supplementary Figures 4-5). BT474 did not express high levels of CD44 on their surface, so we were unable to assess changes within this population (Supplementary Figure 6). Together, these data suggest that the combination of vorinostat and lapatinib can reduce the CSC population.
ii Combination of Vorinostat and Lapatinib Reduces Expression of Self-Renewal Proteins
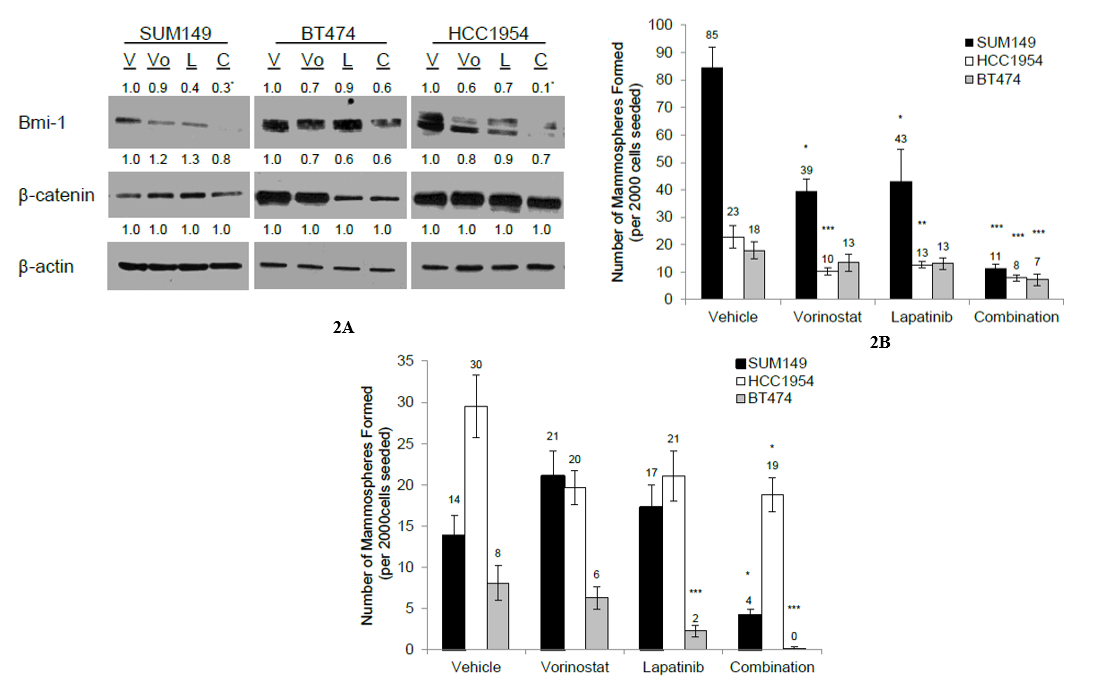
Coupled with the ability to decrease CSC population, it was of interest to determine whether the combination could target self-renewal through the downregulation of pluripotency proteins. SUM149, HCC1954, and BT474 were treated with vehicle, vorinostat or lapatinib, or the combination, for 72 hours, and the effect on the expression of different pluripotency proteins was examined. When treated with the combination, all three cell lines displayed reduced expression of BMI-1, a protein involved in maintaining self-renewal capability (p<0.05 [SUM149], p<0.01 [HCC1954] vs. vehicle-treated control, Figure 2A). The expression of β–catenin, a protein involved with pluripotency, was reduced in all three cell lines following treatment with the combination (Figure 2A). Treatment with either single agent alone had little effect on the expression of β–catenin. Together, these data suggest that the combination inhibits regulators of pluripotency.
Figure 2:
iii Combination of Vorinostat and Lapatinib Inhibits Self-Renewal
As the combination was able to inhibit the expression of different pluripotency proteins, it was of interest to determine whether the combination could also inhibit self-renewal. All three cell lines were treated with vehicle, single agents, or the combination for 72 hours, and 2,000 viable cells were seeded in the mammosphere assay. Treatment with the combination was able to reduce mammosphere formation in all three cell lines significantly (p<0.001 vs. vehicle-treated control, Figure 2B, Supplementary Figure 3). To assess the effect of the combination on self-renewal, the mammospheres from (Figure 2B) were passaged and reseeded under non-adherent conditions in the absence of treatment. A significant reduction in mammosphere formation, which resulted from the combination treatment, was observed (p<0.05 [SUM149, HCC1954], p<0.001 [BT474] vs. vehicle-treated control, Figure 2C). Together, these data suggest that the combination can reduce self-renewal capacity of HER2-positive breast cancer cells.
iv Combination of Vorinostat and Lapatinib Modulates Expression of EMT Markers
Due to the close link between CSC and EMT as described, we further investigate the effects of this combination on EMT and metastasis. HER2 overexpressing cells were treated for 72 hours with the vehicle, 1 µM vorinostat, 1 µM lapatinib, or the combination, and the resulting cells were assayed for changes in epithelial and mesenchymal proteins, including cytokeratin 8/18 (CK8/18, an epithelial marker), TWIST1, and vimentin (mesenchymal markers).
Differential changes were observed amongst the three cell lines; SUM149 exhibited a significant increase in CK8/18 expression following treatment with vorinostat and the combination (p<0.05 vs. vehicle-treated control, Figure 3A), while both HCC1954 and BT474 showed a significant increase in CK8/18 expression following treatment with lapatinib and the combination (p<0.01 lapatinib vs. vehicle in HCC1954, p<0.05 combination vs. vehicle in HCC1954, p<0.05 vs. vehicle-treated control in BT474, Figure 3A). In addition, mesenchymal marker TWIST1 (a transcription factor involved in the regulation of the EMT) was modulated in the basal HER2 cell lines, though differently in each cell line. In SUM149, all three treatment arms significantly reduced the expression of TWIST1 (p<0.01 [vorinostat], p<0.05 [lapatinib], and p<0.001 [combination] vs. vehicle-treated controls, Figure 3A), while only lapatinib and the combination were able to significantly reduce TWIST1 expression (p<0.05 vs. vehicle-treated control). BT474, which is a more epithelial cell line, did not express TWIST1 at the basal level.
All three treatment arms significantly reduced the expression of vimentin, a cytoskeletal protein expressed in mesenchymal cells, in SUM149 cells (p<0.01 [vorinostat], p<0.001 [lapatinib and combination] vs. vehicle-treated controls, Figure 3A). Neither BT474 nor HCC1954 expressed vimentin. Together, these data suggest that vorinostat and lapatinib have differing effects on the expression of epithelial and mesenchymal markers, which is cell line dependent.
Figure 3:
v Combination of Vorinostat and Lapatinib Modulates Morphology of SUM149
As the greatest effect of the combination on epithelial and mesenchymal proteins was observed in the SUM149 cell line, we next observed the effect of the combination on the morphology of the SUM149 cells. Cells were treated for 72 hours and imaged using phase contrast. Following treatment with vorinostat, SUM149 cells became more flat and large when compared to vehicle-treated cells (Figure 3B), while treatment with lapatinib flattened the cells, but did not cause enlargement (Figure 3B). Treatment with the combination resulted in flattening similar to that of the cells treated with lapatinib, and enlargement of the cells in between that observed with vorinostat and lapatinib treatment (Figure 3B). These results suggest that treatment with these compounds increases the epithelial characteristics of the cells, which correlates with increased CK8/18 expression observed in (Figure 3A).
vi Combination of Vorinostat and Lapatinib Inhibits Migratory Potential
Passage through EMT is one of the first steps of the metastatic cascade, and as the combination modulates expression proteins involved in this process, we examined the effect of this combination on migration. Two different methods to assess migratory potential, including qualitative measure using Boyden chamber assay (Figure 3C) and quantitative method using xCELLigence (Figure 3D) were used.
HCC1954 cells were treated for 72 hours with vorinostat, lapatinib, or the combination, and seeded in the top of a Boyden chamber in serum-free conditions in the presence of 0.1% BSA. The Boyden chamber was submerged in media containing either 0.1% BSA (negative control) or 5% FBS. The cells were allowed to migrate for 40 hours, upon which they were fixed, stained with crystal violet, and imaged. HCC1954 treated with vehicle migrating towards 0.1% BSA showed no migration (Supplementary Figure 1), while those migrating toward 5% BSA showed migratory potential that was inhibited following treatment with both vorinostat and lapatinib (Figure 3C). The combination of vorinostat and lapatinib abrogated migration (Figure 3C). The migratory potential of BT474 was also examined via Boyden chamber assay.
Though a similar trend was observed (Supplementary Figure 2), the BT474 cell line showed little migratory potential, possibly due to its epithelial character. The migration of the SUM149 cells was measured via xCELLigence, which measures migration via changes in electrical impedance. A trend similar to that of the HCC1954 cell line was observed in the SUM149 cell line following treatment with vorinostat and lapatinib; vorinostat inhibited migration, but only lapatinib as a single agent was significantly different from the vehicle-treated control (p<0.001, Figure 3D). The combination of lapatinib and vorinostat was able to significantly inhibit the migration of SUM149 (p<0.001 vs. vehicle-treated control), though this was not significantly different from lapatinib alone. Together, these results suggest that the combination of vorinostat and lapatinib is able to decrease the migratory potential of HER2-positive breast cancer cells.
II Phase I/II Clinical Trial
i Patient Characteristics
A total of 12 patients were enrolled, 6 patients in the phase I part, and an additional 6 patients in the phase II part. The first 3 patients were treated at DL1, and 9 additional patients were treated at DL2. Patient characteristics were summarized in (Table 1). The median age was 52 years. All of the patients were female, and 75% (9 out of 12) were African American. Most patients (83%) were diagnosed with breast cancer. The majority (67%) were HER2-positive, including 2 patients in the phase I part and all 6 patients in the phase II part. The preponderance of patients received multiple lines of prior therapy with the median of 3 prior lines (range 1-8). Most of the patients received combination chemotherapy in their previous lines of treatments with the median of 5 prior chemotherapies (range 2-10).
Table 1: Baseline characteristics of the study patients.
|
Patient # |
Age |
Gender |
Race |
PS |
Primary Tumor |
Histology |
ER/PR |
HER2 |
Prior Rx/Line |
|
Dose Level 1 (1250 mg lapatinib + 300mg 4 days on, 3 days off vorinostat) |
|||||||||
|
1 |
53 |
Female |
B |
0 |
Breast |
IDC |
Neg |
Neg |
5/4 |
|
2 |
25 |
Female |
B |
0 |
Breast |
IDC/ Inflammatory |
Pos |
Pos |
3/1 |
|
3 |
51 |
Female |
W |
0 |
Breast |
IDC |
Pos |
Neg |
10/8 |
|
Dose Level 2 (1250 mg lapatinib + 400mg 4 days on, 3 days off vorinostat) |
|||||||||
|
4 |
63 |
Female |
W |
0 |
Thyroid |
Anaplastic |
N/A |
N/A |
3/1 |
|
5 |
50 |
Female |
B |
1 |
Lung |
Squamous |
N/A |
N/A |
5/3 |
|
6 |
66 |
Female |
B |
1 |
Breast |
IDC |
Neg |
Pos |
9/6 |
|
7 |
47 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
8/5 |
|
8 |
52 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
4/1 |
|
9 |
66 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
6/3 |
|
10 |
52 |
Female |
B |
1 |
Breast |
IDC |
Pos |
Pos |
3/1 |
|
11 |
66 |
Female |
W |
1 |
Breast |
IDC |
Pos |
Pos |
2/1 |
|
12 |
42 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
5/4 |
Race: W: white, B: black or African ancestry, Histology: IDC: invasive ductal carcinoma, Pos: positive, Neg: negative.
ii Adverse Events
A total of 6 patients were treated in the phase I part. No DLTs were observed in DL1 or DL2. The recommended phase II dose was vorinostat 400 mg for 4 days on and 3 days off in combination with lapatinib 1,250 mg continuously. Treatment-related AEs in both phase I and phase II parts are listed in (Table 2). Most AEs were grade 1 or 2. Overall, the most common AEs included diarrhea (50%), nausea (41.67%), and fatigue (41.67%). There was 1 grade 3 diarrhea at DL2, which resolved in less than 2 days after diphenoxylate and atropine was started, and 1 grade 3 neutropenia at DL1, which resolved in less than 7 days with dose interruption of vorinostat. One patient discontinued vorinostat in DL2 due to diarrhea, but remained on single agent lapatinib after achieving PR. There was no grade 4 toxicity and no treatment-related death.
Table 2: Adverse events reported anytime during the study.
|
|
|
CTCAE Grade |
|||||
|
Adverse events |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
All grades (%) |
||
|
Nausea |
1 |
3 |
1 |
0 |
5 |
(41.67%) |
|
|
Diarrhea |
3 |
2 |
1 |
0 |
6 |
(50%) |
|
|
Neutropenia |
0 |
1 |
1 |
0 |
2 |
(16.67%) |
|
|
Fatigue |
4 |
1 |
0 |
0 |
5 |
(41.67%) |
|
|
Rash |
2 |
1 |
0 |
0 |
3 |
(25%) |
|
|
Muscle cramps |
2 |
0 |
0 |
0 |
2 |
(16.67%) |
|
|
Elevated ALT |
2 |
0 |
0 |
0 |
2 |
(16.67%) |
|
|
Pruritus |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Stomatitis |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated AST |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Vomiting |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Leukopenia |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Prolonged QTc |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Ocular discharge |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Anemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Headache |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Anorexia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Weight loss |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hypokalemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hyperbilirubinemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hyperglycemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated uric acid |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated creatinine |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
iii Efficacy
All 7 patients with HER2-positive breast cancer treated with the recommended phase II dose were assessed for tumor response. One patient (14%) achieved PR, and 2 patients (29%) had SD. Therefore, the CBR was 43%. The patient who had PR discontinued vorinostat as described and remained on single agent lapatinib. Intriguingly, all 8 patients with HER2-positive breast cancer treated in both DL1 and DL2 did not develop new sites of metastasis while they were treated with the combination of vorinostat and lapatinib. All patients, except one, had progressive disease from enlargement of previously existing target lesions of > 20%. One patient who developed a new lesion had treatment interruption prior to developing progressive disease due to hospitalization from respiratory syncytial virus pneumonia.
Discussion
Our preclinical and clinical studies demonstrate that a pan-HDACi, vorinostat, in combination with lapatinib, may decrease CSCs and reduce metastatic potential. In our preclinical model, the combination of vorinostat and lapatinib significantly reduced CSCs, measured by CSC cell surface markers, including CD49f and CD44+/CD24-/low, as well as ALDH activity. ALDH1 is an enzyme that catalyzes the oxidation of aldehydes. Ginesteir et al. had previously shown that high ALDH1 activity in breast cancer correlates with CSC population, self-renewal capability, and the ability to recapitulate the heterogeneity of the parental tumor [13]. Furthermore, high ALDH expression in primary tumors also correlates with poor outcome in patients with breast cancer. In our study, we observed a more significant reduction of ALDH+ population with the combination compared to either agent alone.
In addition to the CSC phenotypes, the combination also reduced self-renewal protein expression, including Bmi1 and β-catenin. In line with this observation, we also observed a significant reduction in mammosphere formation with the combination treatment. Mammospheres are three-dimensional mammary organoids grown in suspension that are enriched in mammary stem/progenitor cells capable of self-renewal and multi-lineage differentiation [16]. This assay has been used to quantitate stem cell self-renewal capability. Similar to what we observed with reduction in CSC phenotypes, these effects on self-renewal capacity of CSCs were also more prominent with the combination of vorinostat and lapatinib compared to the single agents.
Given the fact that CSCs have been shown to be directly linked to EMT and metastasis, we further investigated and found that the combination of vorinostat and lapatinib significantly reduced mesenchymal markers, TWIST1, and vimentin, as well as increased epithelial marker CK8/18. Corresponding to the changes in EMT-related protein expression, we also observed morphological changes with the combination treatment from mesenchymal spindle cell shape to epithelial flat cell shape. This epithelial morphological change was also observed with single agent vorinostat treatment, but not with lapatinib. In addition, we also evaluated the ability of tumor cells to migrate using xCELLigence. This technology allowed us to monitor migration and invasion of treated live cells. While single agent lapatinib also reduced cell migration, the combination of vorinostat and lapatinib completely abrogated the migration.
Based on these intriguing findings, we conducted a phase I/II trial of vorinostat in combination with lapatinib. The majority of the study participants had advanced HER2-positive breast cancer and had been exposed to multiple lines of prior therapy. Our clinical trial demonstrated that addition of vorinostat to lapatinib is feasible and safe with manageable side effects. Our safety results are in line with other previous studies of these two agents. Consistent with lapatinib side effects, 50% of patients reported diarrhea of any grade with our combination of lapatinib and vorinostat. This percentage of all-grade diarrhea is comparable to previous clinical trials of single-agent lapatinib at the dose of 1,000-1,500 mg per day, which reported all-grade diarrhea ranging from 36% to 57% [17-20].
Similar to what was previously reported with vorinostat, we also observed cytopenia with 16% all-grade neutropenia, including 1 patient with grade 2 and another patient with grade 3 neutropenia. However, in these 2 patients, neutropenia resolved rapidly during the 3-day off period without requiring a dose reduction. In addition, we did not observe increased cardiac toxicity with the combination of vorinostat and lapatinib in our small cohort of patients. There was neither symptomatic congestive heart failure nor clinically significant asymptomatic left ventricular ejection fraction reduction observed.
The CBR of our combination was 43% compared to 31-35% previously reported with single-agent lapatinib in HER2-positive breast cancer [4, 19]. More intriguingly, we observed that patients who continued on vorinostat and lapatinib did not develop any new site of metastasis. This observation confirms our preclinical findings, which showed that the combination of vorinostat and lapatinib treatment decreases the CSC population and prevents metastasis while on therapy. This clinical observation supports our preclinical findings, which showed that the combination of vorinostat and lapatinib decreases the CSC population and prevents metastasis. Due to the small sample size, future clinical trials are needed to confirm these findings.
In the current practice and clinical trials, clinicians use the RECIST 1.1 criteria to determine responses to therapies and investigating agents [11]. Patients can remain on their present treatments as long as they do not have progressive disease. Currently, progressive disease is defined by either at least 20% increase in the size of existing target lesions or the emergence of one or more new lesions. Given the fact that CSCs represent only a small fraction of tumor cells, CSC targeted therapies may not affect the bulk of proliferating tumor cells, and progressive disease from enlargement of existing lesions may lead to discontinuation of therapy. Therefore, novel clinical trial endpoints and correlative studies to assess this small subpopulation of tumor cells are needed to evaluate new investigational agents targeting CSCs. Perhaps, combination therapies that target both CSCs and the bulk population are needed in order to eradicate tumors.
In summary, our preclinical and clinical data suggest that the combination of vorinostat and lapatinib can target the CSC population and prevent new metastasis. Additional studies are needed to validate these results further. Moreover, novel clinical trial endpoint and correlative studies are needed in order to assess new investigational agents targeting the CSC population.
Acknowledgements
For preclinical studies, Merck & Co., Inc. provided drug support for vorinostat. For clinical trial, Merck & Co. and GlaxoSmithKline provided both funding and drug support. Dr. Stuart Martin, (University of Maryland, Baltimore, Maryland) provided SUM149 cell line.
Conflicts of Interest
Dr. Chumsri received research funding support from Merck and drug support from GSK.
Funding
Susan G. Komen received support for the Cure, Merck & Co., Inc., and GlaxoSmithKline.
Supplementary Figure 1:
Supplementary Figure 2:
Supplementary Figure 3:
Supplementary Figure 4:
Supplementary Figure 5:
Supplementary Figure 6:
Article Info
Article Type
Research ArticlePublication history
Received: Fri 29, May 2020Accepted: Sun 28, Jun 2020
Published: Sat 04, Jul 2020
Copyright
© 2023 Saranya Chumsri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.07.04
Author Info
Amanda Schech Angela Brodie Jane Lewis Katherine Tkaczuk Martin J. Edelman Nancy Tait Saranya Chumsri Ting Bao Vered Stearns
Corresponding Author
Saranya ChumsriDepartment of Hematology/Oncology, Mayo Clinic, Jacksonville, Florida, USA
Figures & Tables
Table 1: Baseline characteristics of the study patients.
|
Patient # |
Age |
Gender |
Race |
PS |
Primary Tumor |
Histology |
ER/PR |
HER2 |
Prior Rx/Line |
|
Dose Level 1 (1250 mg lapatinib + 300mg 4 days on, 3 days off vorinostat) |
|||||||||
|
1 |
53 |
Female |
B |
0 |
Breast |
IDC |
Neg |
Neg |
5/4 |
|
2 |
25 |
Female |
B |
0 |
Breast |
IDC/ Inflammatory |
Pos |
Pos |
3/1 |
|
3 |
51 |
Female |
W |
0 |
Breast |
IDC |
Pos |
Neg |
10/8 |
|
Dose Level 2 (1250 mg lapatinib + 400mg 4 days on, 3 days off vorinostat) |
|||||||||
|
4 |
63 |
Female |
W |
0 |
Thyroid |
Anaplastic |
N/A |
N/A |
3/1 |
|
5 |
50 |
Female |
B |
1 |
Lung |
Squamous |
N/A |
N/A |
5/3 |
|
6 |
66 |
Female |
B |
1 |
Breast |
IDC |
Neg |
Pos |
9/6 |
|
7 |
47 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
8/5 |
|
8 |
52 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
4/1 |
|
9 |
66 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
6/3 |
|
10 |
52 |
Female |
B |
1 |
Breast |
IDC |
Pos |
Pos |
3/1 |
|
11 |
66 |
Female |
W |
1 |
Breast |
IDC |
Pos |
Pos |
2/1 |
|
12 |
42 |
Female |
B |
0 |
Breast |
IDC |
Pos |
Pos |
5/4 |
Race: W: white, B: black or African ancestry, Histology: IDC: invasive ductal carcinoma, Pos: positive, Neg: negative.
Table 2: Adverse events reported anytime during the study.
|
|
|
CTCAE Grade |
|||||
|
Adverse events |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
All grades (%) |
||
|
Nausea |
1 |
3 |
1 |
0 |
5 |
(41.67%) |
|
|
Diarrhea |
3 |
2 |
1 |
0 |
6 |
(50%) |
|
|
Neutropenia |
0 |
1 |
1 |
0 |
2 |
(16.67%) |
|
|
Fatigue |
4 |
1 |
0 |
0 |
5 |
(41.67%) |
|
|
Rash |
2 |
1 |
0 |
0 |
3 |
(25%) |
|
|
Muscle cramps |
2 |
0 |
0 |
0 |
2 |
(16.67%) |
|
|
Elevated ALT |
2 |
0 |
0 |
0 |
2 |
(16.67%) |
|
|
Pruritus |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Stomatitis |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated AST |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Vomiting |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Leukopenia |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Prolonged QTc |
0 |
1 |
0 |
0 |
1 |
(8.3%) |
|
|
Ocular discharge |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Anemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Headache |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Anorexia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Weight loss |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hypokalemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hyperbilirubinemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Hyperglycemia |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated uric acid |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|
|
Elevated creatinine |
1 |
0 |
0 |
0 |
1 |
(8.3%) |
|







References
- Benjamin Beck, Cedric Blanpain (2013) Unravelling cancer stem cell potential. Nat Rev Cancer 13: 727-738. [Crossref]
- Raghu Kalluri, Robert A Weinberg (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420-1428. [Crossref]
- Sendurai A Mani, Wenjun Guo, Mai Jing Liao, Elinor Ng Eaton, Ayyakkannu Ayyanan et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704-715. [Crossref]
- T Nakanishi, S Chumsri, N Khakpour, A H Brodie, B Leyland Jones et al. (2010) Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br J Cancer 102: 815-826. [Crossref]
- Suthinee Ithimakin, Kathleen C Day, Fayaz Malik, Qin Zen, Scott J Dawsey et al. (2013) HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 73: 1635-1646. [Crossref]
- H Korkaya, A Paulson, F Iovino, M S Wicha (2008) HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27: 6120-6130. [Crossref]
- Rabia A Gilani, Armina A Kazi, Preeti Shah, Amanda J Schech, Saranya Chumsri et al. (2012) The importance of HER2 signaling in the tumor-initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat 135: 681-692. [Crossref]
- Xiaoxian Li, Michael T Lewis, Jian Huang, Carolina Gutierrez, C Kent Osborne et al. (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100: 672-679. [Crossref]
- Amanda J Schech, Preeti Shah, Stephen Yu, Gauri J Sabnis, Olga Goloubeva et al. (2015) Histone deacetylase inhibitor entinostat in combination with a retinoid downregulates HER2 and reduces the tumor initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat 152: 499-508. [Crossref]
- Thehang H Luu, Robert J Morgan, Lucille Leong, Dean Lim, Mark McNamara et al. (2008) A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res 14: 7138-7142. [Crossref]
- E A Eisenhauer, P Therasse, J Bogaerts, L H Schwartz, D Sargent et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247. [Crossref]
- John Stingl, Peter Eirew, Ian Ricketson, Mark Shackleton, François Vaillant et al. (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439: 993-937. [Crossref]
- Christophe Ginestier, Min Hee Hur, Emmanuelle Charafe Jauffret, Florence Monville, Julie Dutcher et al. (2007) ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 1: 555-567. [Crossref]
- Muhammad Al Hajj, Max S Wicha, Adalberto Benito Hernandez, Sean J Morrison, Michael F Clarke (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100: 3983-3988. [Crossref]
- Carol Sheridan, Hiromitsu Kishimoto, Robyn K Fuchs, Sanjana Mehrotra, Poornima Bhat Nakshatri et al. (2006) CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8: R59. [Crossref]
- Gabriela Dontu, Wissam M Abdallah, Jessica M Foley, Kyle W Jackson, Michael F Clarke et al. (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17: 1253-1270. [Crossref]
- Kimberly L Blackwell, Harold J Burstein, Anna Maria Storniolo, Hope Rugo, George Sledge et al. (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28: 1124-1130. [Crossref]
- Howard A Burris 3rd, Herbert I Hurwitz, E Claire Dees, Afshin Dowlati, Kimberly L Blackwell et al. (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23: 5305-5313. [Crossref]
- H J Burstein, A M Storniolo, S Franco, J Forster, S Stein et al. (2008) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19: 1068-1074. [Crossref]
- Henry L Gomez, Dinesh C Doval, Miguel A Chavez, Peter C S Ang, Zeba Aziz et al. (2008) Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26: 2999-3005. [Crossref]
