Synthesis, Characterization and Cytotoxic Studies of Benzamide Derivatives of Anacardic Acid using Human Liver Cancer Cells
A B S T R A C T
Naturally occurring anacardic acid based benzamides were reported to show anti-inflammatory and anticancer activities, where we synthesized and characterized by NMR and HRMS analysis and also tested a series of anacardic acid based benzamides and evaluated against the proliferation of human liver cancer cells (HepG2). Among the tested compounds, 6j-m showed good inhibitory activity against HepG2 cells with IC50 values ranging from 78.2-91.9 μM. In conclusion, we herein reported the newer series of an academic acid benzamides for the first time.
Keywords
Anacardic acid, cytotoxic, HepG2, benzamid
Introduction
Cancer is a deadly disease in the world with the highest mortal rate [1]. Based on the source and origin of the cancer cells, they are classified into carcinoma, sarcoma, lymphoma or leukemia [2]. Uncontrolled cell proliferation, anti-apoptosis, angiogenesis, metastasis, and genomic instability are the most common features of all types of cancers [3]. There are many ways to treat cancer such as chemotherapy, radiation therapy, surgery, stem cell transplant, immunotherapy, precision medicine etc. Among them, in chemotherapy small molecules are used to treat cancer via different mechanisms.
Basically, anacardic acid is most active against Streptococcus mutants and Cutibacterium acnes [4]. Besides, it has also showed anticancer activity by inhibition of the histone acetyltransferase in human liver cancer cells, phosphodiesterase-5 inhibition, cytotoxic activity, RANKL-induced osteoclastogenesis inhibitors, fibroblast-like synoriocycle proliferation suppressor and ameliorates collagen-induced arthritis, induces cell apoptosis of prostate cancer through autophagy by ER stess/DAPK3/Akt signalling pathway, attenuates phenylephrine-induced cardiac hypertrophy, polyketide biosynthetic pathway modulator in endopytic fungus-Anteaglonium Sp. FL0768, antidepressant, regulates Wnt4 expression in chicken, VEGF-induced angiogenesis inhibitor, excellent paclitaxel transporter in breast cancer therapy, and anti-convulsant activities [5-17]. Thus, in continuation of our work on developing new anacardic acid based benzamides, we designed and synthesized new compounds, which contains alkoxy benzamides as additive groups that acted as cytotoxic agents in the previous reports [18-26].
Results and Discussion
I Chemistry
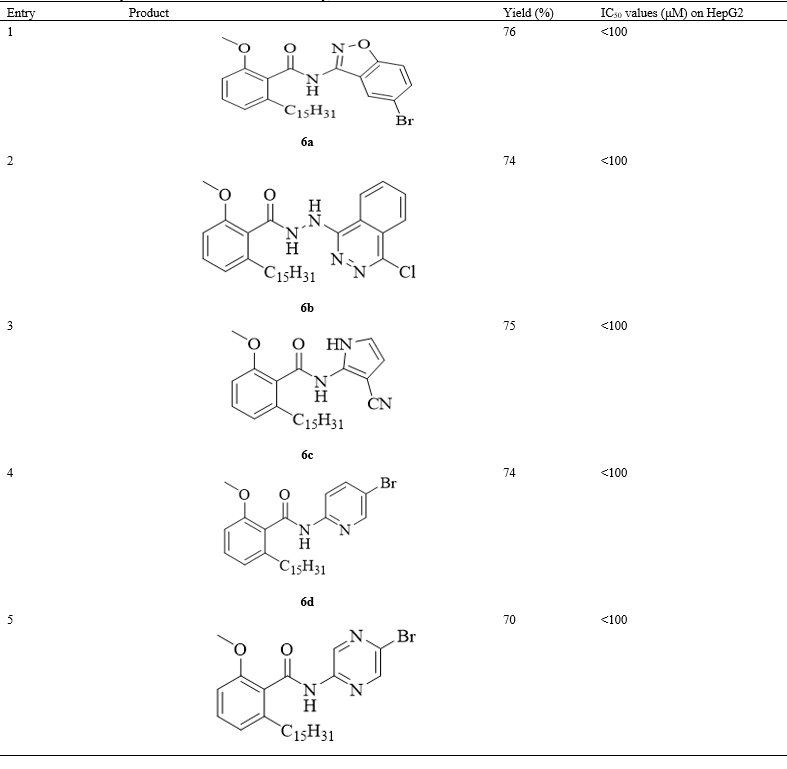
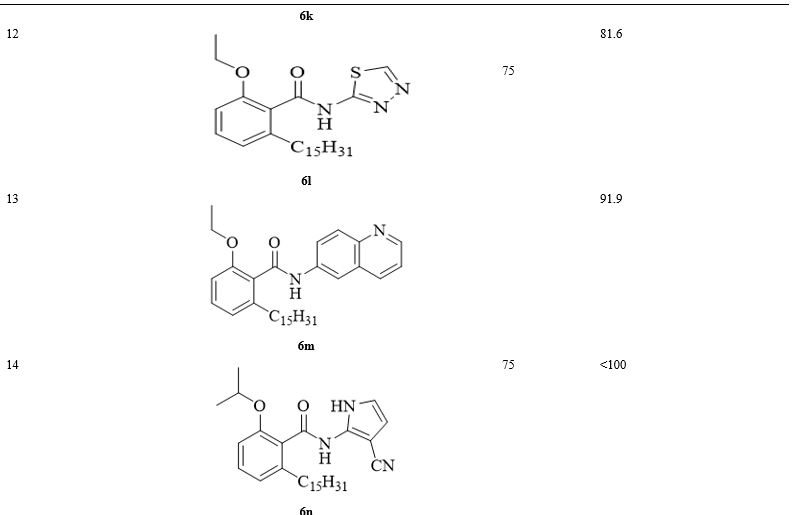
Initially, anacardic acid was isolated according to reported protocol. Further, the liquid was catalytically hydrogenated and purified using column chromatography to obtain pure anacardic acid. Anacardic acid 1 was converted into its corresponding ester 3 by treatment with dimethyl sulphate (2a), diethyl sulphate (2b) and isopropyl chloride (2c). Meanwhile, phenolic-OH was alkylated by these reagents into respective alkoxy groups according to reported methods [6, 28, 29]. Later, alkylated carboxylic acid group was selectively cleaved into carboxylic acid 4 by heating with potassium tertiary butoxide in DMSO. Coupling of carboxylic acid 4 with various amines 5 in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl), hydroxyl benzotriazole (HOBt) and base triethylamine furnished final products: amides of anacardic acid 6. The structures and yields of all title compounds are given in (Table 1). The schematic representation of the synthesis of title compounds were shown in Scheme 1. Further, these compounds were completely characterized by 1H NMR, 13C NMR and IR spectroscopic techniques.
Scheme 1: Synthesis of amides of anacardic acid.


II Biology
Anacardic Acid Derivatives Decreased the Proliferation of Human Liver Cancer Cells (Hepg2)
Using a basic MTT colorimetric assay, we carried out the anti-proliferative effect of anacardic acid derivatives on Human liver cancer cells (HepG2) and it was found that the compounds 6j, 6k, 6l and 6m significantly decreased the proliferation of HepG2 cells with an IC50 values of 86.8, 78.2, 81.6 and 91.9 μM respectively (Table 1). The structure activity relationship studies indicated that anacardic acids with methoxy substituents on benzene ring are inactive against HepG2 cells (<100μM). While, their analogues with ethoxy substituent are active (6j-m) with aforementioned IC50 values. While isobenzofuranone, phenyl-thiadiazole, 3-brommopyridine and 3-cyano-pyrrole were inactive (<100μM). Finally, anacardic acid derivative with isopropyloxy group is also inactive (<100μM).
Experimental Section
I General Methods/ Materials/Instrumentation
CNSL was purchased from commercial source and all other reagents are of analytical grade purchased from Aldrich. The compounds were analysed and confirmed through 1H and 13C NMR spectra using CDCl3/DMSO-d6 as a sovlent at 400 & 100MHz respectively on a Bruker A.G Spectrometer. Chemical Shifts were recorded using tetra methyl silane (TMS) as an internal standard. Nicolet avatar 320 FT-IR spectrometer was used to record IR spectra. Acro Steel Pvt. Ltd. melting point apparatus was used to determine melting points.
II General Procedure for the Preparation of Final Compounds 6
2-alkoxy-6-pentadecylbenzoic acid 4 (0.6 mmol) was dissolved in dichloromethane (5 mL) using a magnetic stirrer and cooled to 0°C . To that EDC.HCl (0.6 mmol), HOBt (0.6 mmol) and amine 5 (0.6 mmol) were added. The completion of the reaction was monitored through TLC and neutralized with dilute HCl (25 mL) extracted with ethyl acetate (25 mL x 3), washed with water and brine (25 mL) and dried over sodium sulphate and concentrated. The obtained crude title compounds were purified by column chromatography using silica gel (60-120 mesh) and 0-5% ethyl acetate in petroleum ether as an effluent to procure white solids of pure title compounds.
III N-(5-Bromobenzo(d) isoxazol-3-yl)-2-methoxy-6pentadecyl benzamide (6a)
White solid. 76%. m.p. 85-87 ° C; 1H NMR (DMSO, 400MHz): δ 0.827 (t, J=7.2 Hz, 3H, CH3), 1.17 (m, 24H, (CH2)12), 1.61 (m, 2H, CH2), 2.70 (m, 2H, CH2), 4.00 (s, 3H, OCH3), 7.0 Hz (d, J =7.6 Hz, 1H, Ar-H), 7.15 (d, J=8.4 Hz, 1H, Ar-H), 7.55 (m, 2H, Ar-H), 7.65 (d, J=8.0 Hz, 1H, Ar-H), 7.7 (t, J=7.6 Hz ,1H, Ar-H), 8.1 (d, J=8.4 Hz, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.6, 108.7, 120.3, 120.5, 120.8, 122.0, 124.7, 128.5, 128.7, 132.6, 143.6, 143.6, 157.5, 164.3; HRMS (ESI-TOF) m/z: [C30H41BrN2O3], 557.2379 and 559.2358
IV N-(4-Chlorophthalazin-1-yl-2-methoxy-6-pentadecyl benzohydrazide (6b)
White solid. 74%. m.p. 84-86 ° C; 1H NMR (DMSO, 400 MHz): δ 0.81 (t, J=6.4Hz, 3H, CH3), 1.17 (m, 24H, (CH2)12), 1.61 (m, 2H, CH2), 2.7 (t, J=7.6Hz, 2H, CH2), 4.00 (s, 3H, OCH3), 6.56 (s, 1H, Ar-H) 7.0 Hz (d, J=7.6 Hz, 1H, Ar-H), 7.15 (d, J=8.4Hz, 1H, Ar-H), 7.55 (m, 2H, Ar-H, NH), 7.65 (d, J=8.0 Hz, 1H, Ar-H), 7.7 (t, J=7.6Hz, 1H, Ar-H), 8.18 (m, 2H, Ar-H, NH); 13C NMR (CDCl3, 100MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.6, 108.7, 120.3, 120.5, 120.8, 122.0, 124.7, 128.5, 128.7, 132.6, 143.6, 143.6, 157.5, 158.0, 164.3; HRMS (ESI-TOF) m/z: C31H44ClN4O2, 539.3153.
V N-(-3-Cyano-1H-pyrrol-2-yl)-2-methoxy-6-pentadecyl benzamide (6c)
White solid. 75%. m.p. 82-85 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8 Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.70 (m, 2H, CH2), 2.7 (t, J=7.6 Hz, 2H, CH2), 4.00 (s, 3H, OCH3) 6.9 (d, J=8.4 Hz, 1H, Ar-H), 6.9 (d, J=8.0 Hz, 1H, Ar-H), 7.44 (m, 2H, Ar-H), 7.57 (m, 2H, Ar-H, NH), 8.09 (m, 1H, NH); 13C NMR (CDCl3, 100MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.6, 108.7, 120.3, 120.5, 120.8, 122.0, 124.7, 128.5, 128.7, 143.6, 157.5, 158.0, 164.3; HRMS (ESI-TOF) m/z: C28H42N3O2, 452.3277
VI N-(5-Bromopyridin-2-yl)-2-methoxy-6 pentadecylbenzamide (6d)
White solid. 74%. m.p. 80-82 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8 Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.68 (m, 2H, CH2), 2.7 (t, J=7.6 Hz, 2H, CH2), 4.00 (s, 3H, OCH3), 6.89 (d, J=8.4 Hz, 1H, Ar-H), 6.96 (d, J=7.6Hz, 1H, Ar-H), 7.42-7.48 (m, 2H, Ar-H), 7.57-7.58 (m, 2H, Ar-H, NH), 8.09 (d, J=8.4 Hz, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.6, 117.4, 120.4, 122.0, 124.7, 128.5, 128.72, 132.6, 143.6, 143.6, 157.5, 164.3; HRMS (ESI-TOF) m/z:C28H42BrN2O2,517.2430 and 519.2409
VII N-(5-Bromopyrazin-2-yl)-2-methoxy-6-pentadecylbenzamide (6e)
White solid. 70%. m.p. 80-82 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.70 (m, 2H, CH2), 2.7 (t, J=7.6, 2H, CH2), 4.00 (s, 3H, OCH3), 6.90 (d, J=8.4 Hz, 1H, Ar-H), 6.96 (d, J=8.0 Hz, 1H, Ar-H), 7.44 (m, 2H, Ar-H, NH), 7.58 (d, J=3.6 Hz, 1H, Ar-H), 8.09 (d, J=8.4, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): 13C NMR (CDCl3, 100MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.6, 108.7, 117.4, 120.4, 122.0, 124.7, 218.5, 132.6, 143.6, 157.5, 164.3; HRMS (ESI-TOF) m/z: C27H41BrN3O2,518.2382 and 520.2362
VIII 2-Ethoxy-N-(-1-oxo-1,3,dihydroiso benzofuran-5-yl)-6-pentadecylbenzamide (6f)
White solid. 75%. m.p. 86-88 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.4 Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (t, J=7.2 Hz, 3H, CH3), 1.70 (m, 2H, CH2), 2.78 (t, J=8.0 Hz, 2H, CH2), 4.20 (q, 2H, (OCH2)2, 6.87 Hz (d, J=8.4 Hz, 1H, Ar-H), 6.94 (d, J=7.6 Hz, 1H, Ar-H), 7.41 (m, 2H, Ar-H), 7.75 (m, 2H, Ar-H), 7.61 (m, 1H, Ar-H), 8.09 (d, J=8.4, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): 13C NMR (CDCl3, 100MHz): δ 14.0, 14.9, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 64.8, 108.71, 109.56, 120.45, 121.78, 124.66, 128.41, 128.78, 132.52, 143.51, 143.55, 156.89,164.41; HRMS (ESI-TOF) m/z:C32H46NO4, 508.3427.
IX 2-Ethoxy-6-pentadecyl-N-(5-phenyl-1,3,4-thiadiazol-2-yl-) benzamide (6g)
Yellow solid. 75%. m.p. 67-69 ° C; 1H NMR (CDCl3, 400 MHz); δ 0.85 (t, J=7.2Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (t, J=7.2 Hz, 3H, CH3), 1.68 (m, 2H, CH2), 2.7 (t, J=8.0 Hz, 2H, CH2), 4.20 (m, 2H, OCH2), 6.87 (d, J=8.4Hz, 1H, Ar-H), 6.94 (d, J=7.6Hz, 1H, Ar-H), 7.41 (m, 2H, Ar-H), 7.54 (m, 1H, Ar-H), 7.4 (m, 2H, Ar-H), 8.09 (d, J=8.4, 1H, Ar-H); 13C NMR (CDCl3,100MHz): δ 14.0, 14.8, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 56.2, 108.7, 109.6, 109.6, 117.7, 120.5, 121.8, 124.6, 128.4, 128.8, 132.5, 132.5, 143.5, 143.6, 156.9, 164.4; HRMS (ESI-TOF) m/z: C32H46N3O2S, 536.3311.
X N-(3-Bromopyridin-2-yl)-2-ethoxy-6-pentadecyl benzamide (6h)
Yellow solid. 75%. m.p. 82-84 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (m, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.70 (m, 2H, CH2), 2.78 (t, J=8.0 Hz, 2H, CH2), 4.20 (m, 2H, OCH2), 6.87 (d, J=8.4 Hz, 1H, Ar-H), 6.94 (d, J=8.0 Hz, 1H, Ar-H), 7.41-47 (m, 2H, Ar-H), 7.57-7.63 (m, 2H, Ar-H, NH), 8.10 (d, J=8.8 Hz, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): δ 14.0, 14.9, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 64.8, 108.7, 109.6, 117.7, 120.5, 121.8, 124.6, 126.5, 128.4, 132.5, 143.6, 156.9, 164.4; HRMS (ESI-TOF) m/z: C29H44BrN2O2, 531.2586.
XI N-(3-Cyano-3H-pyrrol-2-yl)-2-ethoxy-6-pentadecyl benzamide (6i)
White solid. 75%. m. p. 90-92 ° C; 1H NMR (CDCl3, 400MHz): δ 0.85 (m, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.68 (m, 2H, CH2), 2.78 (t, J=8.0 Hz, 2H, CH2), 4.19 (m, 2H, OCH2), 6.87 (d, J=8.0 Hz, 1H, Ar-H), 6.94 (d, J=7.6 Hz, 1H, Ar-H), 7.41 (m, 1H, Ar-H), 7.55 (m, 1H, Ar-H), 8.09 (d, J=8.0 Hz, 1H, Ar-H); 13C NMR (CDCl3,100 MHz): δ 14.0, 14.9, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 64.8, 109.2, 110.0, 118.0, 120.9, 122.2, 125.2, 128.9, 129.2, 133.0, 143.9, 157.3, 164.9; HRMS (ESI-TOF) m/z: C29H44N3O2, 466.3434.
XII 2-Ethoxy-N-(3-hydroxy phenyl)-6-pentadecyl benzamide (6j)
White solid. 70%. m.p. 87-89 ° C; 1H NMR (CDCl3, 400MHz) δ 0.85 (m, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.68 (m, 2H, CH2), 2.78 (t, J=8.0 Hz, 2H, CH2), 4.20 (m, 2H, OCH2), 6.87 (d, J=8.4 Hz, 1H, Ar-H), 6.94 (d, J =7.6 Hz, 1H, Ar-H), 7.41 (m, 2H, Ar-H), 7.57 (m, 1H, Ar-H), 7.61 (m, 1H, Ar-H), 8.09 (d, J=8.4, 1H, Ar-H); 13C NMR (CDCl3, 100 MHz):14.0, 14.8, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (three peaks), 29.6, 31.3, 31.9, 33.7, 64.8, 108.7, 109.6, 120.4, 120.4, 121.8, 124.6, 128.4, 128.4, 132.5, 143.5, 143.6, 156.9, 164.4; HRMS (ESI-TOF) m/z: C30H46NO3, 468.3478
XIII N-(5-Bromopyrazin-2-yl)-2-ethoxy-6-pentadecyl benzamide (6k)
Yellow solid. 75%. m.p.82-84 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8 Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.68 (m, 2H, CH2), 2.77 (t, J=8.0 Hz, 2H, CH2), 4.2 (m, 2H, OCH2), 6.87 Hz (d, J=8.4 Hz, 1H, Ar-H), 6.94 (d, J=7.6 Hz, 1H, Ar-H), 7.41 (m, 1H, Ar-H), 7.57 (m, 2H, Ar-H, NH), 8.09 (d, J=8.4 Hz, 1H, Ar-H); 13C NMR (CDCl3, 100MHz): δ 14.0, 14.8, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 64.8, 108.7, 109.6, 117.8, 120.5, 121.8, 124.6, 128.4, 132.5, 143.5, 156.9, 164.4; HRMS (ESI-TOF) m/z: C28H13BrN3O2,532.2539 and 534.2520
XIV 2-Ethoxy-6-pentadecyl-n-(1,3,4-thiadiazol-2-yl)benzamide (6l)
White solid. 75%. m.p. 85-87° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.7 (m, 2H, CH2), 2.77 (t, J=8.0 Hz, 2H, CH2), 4.2 (m, 2H, OCH2), 6.94 (d, J=7.6Hz, 1H, Ar-H), 7.41 (m, 1H, Ar-H), 7.55 (m, 1H, Ar-H), 7.61 (s, 1H, NH), 8.09 (d, J=8.4 Hz, 1H, Ar-H); 13C NMR (CDCl3, 100 MHz) δ 14.0, 14.9, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 64.8, 108.7, 109.6, 120.4, 121.8, 124.5, 132.5, 143.5, 156.9, 164.4; HRMS (ESI-TOF) m/z:C26H42N3O2S, 460.2998.
XV 2-Ethoxy-6-pentadecyl-N-(quinolin-6-yl)benzamide (6m)
Yellow solid. 75%. m.p.65-67 ° C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (t, J=6.8Hz, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.53 (m, 3H, CH3), 1.70 (m, 2H, CH2), 2.78 (t, J=8Hz, 2H, CH2), 4.2 (m, 2H, OCH2), 6.87 (d, J=8.4 Hz, 1H, Ar-H), 6.94 (d, J=7.6Hz, 2H, Ar-H), 7.41 (m, 2H, Ar-H), 7.54 (m, 2H, Ar-H), 7.61 (m, 1H, Ar-H), 8.09 (d, J=8.4Hz, 1H, Ar-H); 13C NMR (CDCl3, 50MHz): δ 14.0, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 32.5, 33.7, 64.8, 108.7, 109.6, 117.7, 120.5, 121.8, 124.6, 128.4, 128.8, 132.5, 143.5, 143.6, 155.0, 156.9, 164.4; HRMS (ESI-TOF) m/z: C33H47N2O2, 503.3638
XVI N-(3-Cyano-1-H-pyrrol-2-yl)-2-isopropoxy-6-pentadecyl benzamide (6n)
White solid. 75%. m.p. 63-65°C; 1H NMR (CDCl3, 400 MHz): δ 0.85 (m, 3H, CH3), 1.23 (m, 24H, (CH2)12), 1.45 (d, J=6.0 Hz, 6H, (CH3)2), 1.68 (m, 2H, CH2), 2.77 (t, J=8.0 Hz, 2H, CH2), 4.72 (m, 1H, CH), 6.89 (d, J=8.4 Hz, 1H, Ar-H), 6.92 (d, J=7.6Hz, 1H, Ar-H), 7.40 (m, 2H, Ar-H), 7.55 (s, 1H, NH), 7.61 (m, 1H, NH), 8.09 (d, J=8.4, 1H, Ar-H); 13C NMR (CDCl3, 100 MHz): δ 14.0, 22.1, 22.6, 29.3 (two peaks), 29.4 (two peaks), 29.5 (two peaks), 29.6 (two peaks), 29.6 (two peaks), 31.3, 31.9, 33.7, 71.7, 108.7, 110.9, 118.9, 120.5, 121.6, 124.6, 128.3, 128.8, 132.3, 143.5, 143.6, 155.9, 164.4; HRMS (ESI-TOF) m/z: C30H46N3O2, 480.3590
XVII In Vitro Cytotoxicity Assay
The recently synthesized derivatives were assessed for toxicity against hepatocellular carcinoma (HepG2) cell lines as reported earlier [30-32]. Briefly, the cells (1 X 104/ml) were transferred to 96-well microtiter plate in with or without novel compounds of different concentration of 0.15ml and incubated at 37oC for 72h. After incubation, 20 μl of MTT (5 mg/ml in PBS) dye was transferred to the wells and incubated for 2h at 37oC. The incubation was continued for 24h after the addition of 0.1ml lysis buffer (20% SDS, 50%DMF) and their obsorbance was measured using a microplate reader at a wavelength of 570nm.
Conclusion
In conclusion, the toxicity of newly synthesized benzamide derivatives of anacardic acid were tested against human liver cancer cell lines (HepG2). Amongst, 6j-m showed good inhibitory activity with IC50 values 78.2-91.9 μM.
Consent for Publication
All the authors given consent for this publication.
Conflicts of Interest
None.
Funding
None.
Supplementary Material
Supplementary material for this article is available at.
Abbreviations
RANKL: Receptor Activator of Nuclear factor Kappa-3
ER: Estrogen Receptor
DAPK3: Death Associated Protein Kinase 3
VEGF: Vascuar Endothelial Growth Factor
EDC.HCl: 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
HOBt: Hydroxybenzotriazole
NMR: Nuclear Magnetic Resonance
IR: Infra-Red
GCMS: Gas Chromatography Mass Spectra
Article Info
Article Type
Research ArticlePublication history
Received: Tue 07, Apr 2020Accepted: Mon 20, Apr 2020
Published: Mon 27, Apr 2020
Copyright
© 2023 Dinesh Rangappa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.07
Author Info
Basappa . Dinesh Rangappa K.N. Thanuja Kanchugarakoppal S. Rangappa M. Navya Rani Toreshetahally R. Swaroop
Corresponding Author
Dinesh RangappaDepartment of Nanotechnology, Visvesvaraya Technological University, Center for Postgraduate Studies, Bengaluru, India
Figures & Tables





References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E et al. (2011) Global cancer statistics. CA Cancer J Clinicians 61: 69-90. [Crossref]
- Berg JW (1967) The incidence of multiple primary cancers. I. Development of further cancers in patients with lymphomas, leukemias, and myeloma. J Natl Cancer Inst 38: 741-752. [Crossref]
- Khan I, Ibrar A, Abbas N (2014) Oxadiazoles as privileged motifs for promising anticancer leads: Recent advances and future prospects. Arch Pharm (Weinheim) 347: 1-20. [Crossref]
- Kubo I, H Muroi, M Himejima (1993) Structure - Antibacterial activity relationships of anacardic acids. J Agric Food Chem 41: 1016-1019. [Crossref]
- Ghizzoni M, Boltjes A, Graaf Cd, Haisma HJ, Dekker FJ (2010) Improved inhibition of the histone acetyltransferase PCAF by an anacardic acid derivative. Bioorg Med Chem 15: 5826-5834. [Crossref]
- R Paramashivappa, P Phani kumar, PJ Vithayathil, A Srinivasa Rao (2011) Synthesis of Sildenafil analogues from anacardic acid and their phosphodiesterase-5 inhibition. J Agri Food Chem 49: 2548-2551.
- Chandregowda V, Kush A, Reddy GC (2009) Synthesis of benzamide derivatives of anacardic acid and their cytotoxic activity. Eur J Med Chem 44: 2711-2719. [Crossref]
- Zhao K, Jia Y, Peng J, Pang C, Zhang T et al. (2019) Anacardic acid inhibits RANKL-induced osteoclastogenesis in vitro and prevents ovariectomy-induced bone loss in vivo. FASEB J 33: 9100-9115. [Crossref]
- Yang GH, Zhang C, Wang N, Meng Y, Wang YS (2018) Anacardic acid suppresses fibroblast-like synoviocyte proliferation and invasion and ameliorates collagen-induced arthritis in a mouse model. Cytokine 111: 350-356. [Crossref]
- Tan J, Jiang X, Yin G, He L, Liu J et al. (2017) Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Aktsignaling pathway. Oncol Rep 38: 1373-1382. [Crossref]
- Peng C, Luo X, Li S, Sun H (2017) Phenylephrine-induced cardiac hypertrophy is attenuated by a histone acetylase inhibitor anacardic acid in mice. Mol Biosyst 28: 714-724. [Crossref]
- Mafezoli J, Xu YM, Hilário F, Freidhof B, Espinosa-Artiles P et al. (2018) Modulation of polyketide biosynthetic pathway of the endophytic fungus, Anteaglonium sp. FL0768, by copper (II) and anacardic acid. Phytochem Lett 28: 157-163. [Crossref]
- Júnior ALG, Tchekalarova JD, da Conceição Machado K, Silva SWC, Paz MFCJ et al. (2019) Antidepressant-like effect of anacardic acid in mice via the L-arginine-nitric oxide-serotonergic system. Phytother Res 33: 2126-2138. [Crossref]
- Jiang X, Zhang H, Mehmood K, Li K, Zhang L et al. (2019) Effect of anacardic acid against thiram induced tibialdyschondroplasia in chickens via regulation of Wnt4 expression. Animals (Basel) 9: E82. [Crossref]
- Yuan M, Song X, Lv W, Xin Q, Wang L et al. (2019) Effect of anacardic acid against echinococcosis through inhibition of VEGF-induced angiogenesis. Vet Res 50: 3. [Crossref]
- Rege MD, Ghadi R, Katiyar SS, Kushwah V, Jain S (2019) Exploring an interesting dual functionality of anacardic acid for efficient paclitaxel delivery in breast cancer therapy. Nanomedicine (Lond) 14: 57-75. [Crossref]
- Luiz Gomes A Júnior, Dimitrova Tchekalarova J, Atanasova M, da Conceição Machado K, de Sousa Rios MA et al. (2018) Anticonvulsant effect of anacardic acid in murine models: Putative role of GABAergic and antioxidant mechanisms. Biomed Pharmacother 106: 1686-1695. [Crossref]
- Keerthy HK, Mohan S, Basappa, Bharathkumar H, Rangappa S et al. (2019) Triazole-Pyridine Dicarbonitrile Targets Phosphodiesterase 4 to Induce Cytotoxicity in Lung Carcinoma Cells. Chem Biodivers 16: e1900234. [Crossref]
- Mohan CD, Bharathkumar H, Dukanya, Rangappa S, Shanmugam MK et al. (2018) N-Substituted pyrido-1,4-oxazin-3-ones induce apoptosis of hepatocellular carcinoma cells by targeting NF-κB signaling pathway. Front Pharmacol 9: 1125. [Crossref]
- Gilandoust M, Harsha KB, Mohan CD, Raquib AR, Rangappa S et al. (2018) Synthesis, characterization and cytotoxicity studies of 1,2,3-triazoles and 1,2,4-triazolo [1,5-a] pyrimidines in human breast cancer cells. Bioorg Med Chem Lett 28: 2314-2319. [Crossref]
- Y Yuan, HL Ang, X Lai, L Wang, V Pandey et al. (2018) Breaking down all we know about PTK6 in breast. Cancer Preprints 2018080044.
- Mohan CD, Anilkumar NC, Rangappa S, Shanmugam MK, Mishra S et al. (2018) Novel 1,3,4-oxadiazole induces anticancer activity by targeting NF-κB in hepatocellular carcinoma cells. Front Oncol 8: 42. [Crossref]
- Bhat P, Kriel J, Shubha Priya B, Basappa, Shivananju NS et al. (2018) Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochemical Pharmacology 147: 170-182. [Crossref]
- Anusha S, Cp B, Mohan CD, Mathai J, Rangappa S et al. (2015) A Nano-MgO and Ionic Liquid-Catalyzed ‘Green’ Synthesis Protocol for the Development of Adamantyl-Imidazolo-Thiadiazoles as Anti-Tuberculosis Agents Targeting Sterol 14α-Demethylase (CYP51). PLoS One 10: e0139798. [Crossref]
- Sebastian A, V Pandey, CD Mohan, YT Chia, S Rangappa et al. (2016) Novel Adamantanyl-Based Thiadiazolyl Pyrazoles Targeting EGFR in Triple-Negative Breast Cancer. ACS Omega 1: 1412-1424.
- Anilkumar NC, Sundaram MS, Mohan CD, Rangappa S, Bulusu KC et al. (2015) A One Pot Synthesis of Novel Bioactive Tri-Substitute-Condensed-Imidazopyridines that Targets Snake Venom Phospholipase A2. PLoS One 10: e0131896. [Crossref]
- RK Vempati, S Reddy, N Srinivasa Rao, PK Dubey (2011) Synthesis of novel benzylamine analogues of anacardic acid as potent antibacterial agents. Der Pharma Chemica 3: 500-512.
- Chandregowda V, Kush A, Reddy GC (2009) Synthesis of benzamide derivatives of anacardic acid and their cytotoxic activity. Eur J Med Chem 44: 2711-2719. [Crossref]
- Priya BS, Swamy SN, Tejesvi MV, Basappa, Sarala G et al. (2006) Synthesis, characterization, antimicrobial and single crystal X-ray crystallographic studies of some new sulfonyl, 4-chloro phenoxy benzene and dibenzoazepine substituted benzamides. Eur J Med Chem 41: 1262-1270. [Crossref]
- Sulaiman NB, Mohan CD, Basappa S, Pandey V, Rangappa S et al. (2016) An azaspirane derivative suppresses growth and induces apoptosis of ER-positive and ER-negative breast cancer cells through the modulation of JAK2/STAT3 signaling pathway. Int J Oncol 49: 1221-1229. [Crossref]
- Rakesh KS, Jagadish S, Swaroop TR, Mohan CD, Ashwini N et al. (2015) Anti-Cancer Activity of 2,4-Disubstituted Thiophene Derivatives: Dual Inhibitors of Lipoxygenase and Cyclooxygenase. Med Chem 11: 462-472. [Crossref]
