Synchronous Bilateral Solid Papillary Carcinoma of Breast: A Rare Case Report
Synchronous Bilateral Solid Papillary Carcinoma of Breast: A Rare Case Report
A B S T R A C T
Background: Solid papillary carcinoma is a rare type of carcinoma that accounts for less than 1% of all breast cancers and mostly seen in postmenopausal women. This report presents a rare case of synchronous bilateral solid papillary carcinoma of the breast.
Case Report: A 74-year-old female patient had a mass in her right and left breast. Bilateral total mastectomy and sentinel lymph node biopsy were performed. The pathologic diagnosis was synchronous bilateral solid papillary carcinoma. No lymph node metastasis was detected in either of the breasts.
Conclusion: To our knowledge, little is known about simultaneous bilateral solid papillary carcinomas. Solid papillary carcinoma should be kept in mind in the differential diagnosis of bilateral breast masses with neuroendocrine differentiation in elderly patients.
Keywords
Bilateral breast carcinoma, solid papillary carcinoma, old age, breast neoplasm, solid papillary neoplasm, bilateral
Introduction
Solid papillary carcinoma (SPC) is a rare type of carcinoma that accounts for less than 1% of all breast cancers. The majority occurs in postmenopausal women and 7th decade on average. Solid papillary carcinoma is a variant of papillary breast carcinoma consisting of expanding cellular nodules histopathologically located close to each other. Fibrovascular cores are very thin and solid pattern is observed at small magnification. Neuroendocrine differentiation is common. According to tumor diameter, it may occur as clinical, mass or mammographic abnormalities [1]. We aimed to contribute to literature by presenting this rare tumor which can be bilateral in 5% [2].
Case Report
A 74-year-old female patient presented to the breast clinic with a complaint of a mass in her right breast. MRI revealed a type-3 enhancement pattern in the right breast. Irregular contour heterogeneous enhancement of mass was observed in the left breast and histopathological examination was recommended for both breasts. PET-CT showed increased nodular density in the right breast and increased focal and malignant FDG uptake in the left breast, indistinguishable from breast tissue. Tru-Cut biopsies revealed a well-differentiated neuroendocrine tumor in the right breast. In the left breast, breast carcinoma with neuroendocrine differentiation was diagnosed. With these results, bilateral mastectomy was performed.
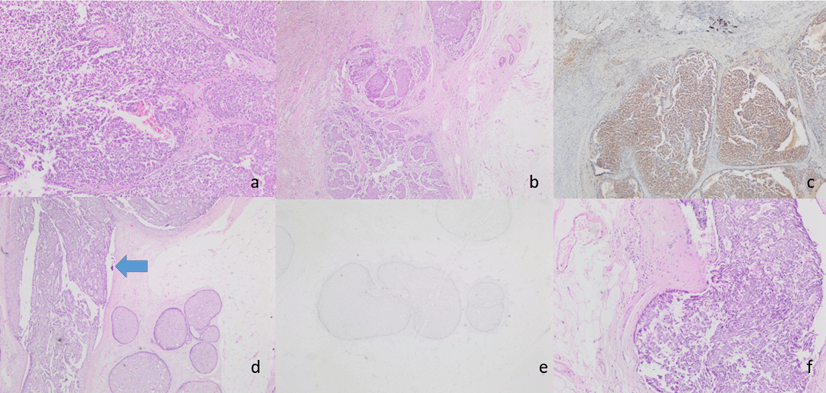
Mastectomy specimens showed a mass lesion of 1.8 cm in diameter in the lower outer quadrant and a solid lesion of 2 cm in the upper middle quadrant in the left breast. Histopathological examination of the lesion revealed fibrovascular structures in irregularly bounded nests in the geographical pattern and no expression was observed around the nests with myoepithelial markers. Immunohistochemically, hormone receptors were positive, HER-2 was negative, and focal neuroendocrine differentiation was detected with chromogranin and synaptophysin and NSE. Mucinous differentiation was not observed. Based on these findings, the patient was diagnosed with solid papillary carcinoma. Sentinel lymph node examination in both axillae showed reactive lymph nodes. In our case, ductal carcinoma in situ (DCIS) nests were also accompanied by solitary papillary carcinoma areas (Figures 1a, 1b, 1c, 1d, 1e & 1f). No recurrence or metastasis was observed within the 2-year follow-up.
Figure 1: a) H&E 100X fibrovascular cores in SPC; b) H&E 20X SPC -well circumscribed solid expansile tumor nodules; c) Tumor cells are showing diffuse positivity for NSE IHC stain; d) SPC nodule (blue arrow), H&E 40X; e) P63 stain x100x; f) SPC -streaming pattern growth and palisading of tumor cells were evident around the fibrovascular cores. H&E 200x.
Discussion
Among all breast cancers bilateral breast carcinomas accounts 2-6 %. Bilateral breast carcinomas is generally defined as synchrone bilateral breast carcinomas when contralateral breast carcinoma is diagnosed within 3 months. If contralateral breast carcinoma is diagnosed more than 3 months, this type of bilateral breast carcinoma is defined as metachronous bilateral breast carcinoma [3]. So our case is defined synchrone bilateral breast carcinoma due to simultaneous diagnosis.
The differential diagnosis includes intraductal papilloma and fluoride ductal hyperplasia. However, these entities do not include fibrovascular cores which are characteristic of solid papillary carcinoma neuroendocrine/mucinous phenotype, cellular homogeneity and mitotic activity [4, 5]. In difficult cases, high molecular weight keratin (such as keratin 5/6) may have diagnostic value; this marker will be positive in benign proliferative lesions [1]. SPC may be confused with papillary DCIS, but the plasmacytoid pattern, mucin production, and fibrotic stroma between ducts support SPC. It may be confused with lobular carcinoma in situ (LCIS), but the presence of cohesive tumor cells and fibrovascular cores supports SPC [6]. Since SPC does not contain myoepithelial layers around the tumor it may be confused with non-specific type invasive carcinoma in the solid pattern [7]. The presence of fibrovascular cores with uniformly circumscribed tumor nodules supports the SPC.
Expression of chromogranin and/or synaptophysin for neuroendocrine differentiation in SPC is observed in at least half of the cases. The tumor cell population is diffuse positive with hormone receptors and HER-2 negative [1]. In accordance with the literature, chromogranin, synaptophysin and hormone receptors were positive while HER-2 was negative in our lesion. To date, a small number of bilateral SPC has been reported in literature [8]. The one in our case contributes to literature. SPC cases may be accompanied by DCIS at a rate of 24% and invasive carcinoma at a rate of 75% [9]. In our case, there were areas of intraductal carcinoma in the solid pattern accompanying SPC.
In conclusion, solid papillary carcinoma should be kept in mind in the differential diagnosis of tumors with mucinous compartment and neuroendocrine differentiation in elderly patients. It may be bilateral and should be clinically and pathologically considered.
Acknowledgments
None.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Sat 29, Aug 2020Accepted: Tue 29, Sep 2020
Published: Tue 10, Nov 2020
Copyright
© 2023 Ayşe Nur Uğur Kılınç. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.OCR.2020.01.01
Author Info
Ayşe Nur Uğur Kılınç Zeynep Bayramoğlu Yaşar Ünlü Nergis Aksoy Mehmet Ali Eryilmaz
Corresponding Author
Ayşe Nur Uğur KılınçPathology, Konya Training and Research Hospital, Konya, Turkey
Figures & Tables
References
- Mac Gorgan G, Collins LC, Lerwill M, Rakha EA, Tan BY (2019) Solid papillary carcinoma. WHO classification of tumours of the breast, 5th edn. Lyon, France: IARC Press 2019:63-64.
- Saremian J, Rosa M (2012) Solid papillary carcinoma of the breast: a pathologically and clinically distinct breast tumor. Arch Pathol Lab Med 136: 1308-1311. [Crossref]
- Nassar H, Qureshi H, Adsay NV, Visscher D (2006) Clinicopathologic analysis of solid papillary carcinoma of the breast and associated invasive carcinoma. Am J Surg Pathol 30: 501‐507. [Crossref]
- Schnitt J, Collins LC (2013) Biopsy interpretation of the breast 2nd edition. Lippincott Williams & Wilkins 2013: 250-254.
- Maluf HM, Koerner FC (1995) Solid papillary carcinoma of the breast. A form of intraductal carcinoma with endocrine differentiation frequently associated with mucinous carcinoma. Am J Surg Pathol 19: 1237‐1244. [Crossref]
- Eliyatkin N, Zengel B, Yagci A, Comut E, Postaci H et al. (2015) Properties of synchronous versus metachronous bilateral breast carcinoma with long time follow up. Asian Pac J Cancer Prev 16: 4921-4926. [Crossref]
- Guo S, Wang Y, Rohr J, Fan C, Li Q et al. (2016) Solid papillary carcinoma of the breast: a special entity needs to be distinguished from conventional invasive carcinoma avoiding overtreatment. Breast 26: 67‐72. [Crossref]
- Yoshimura N, Murakami S, Kaneko M, Sakatani A, Hirabayashi N et al. (2013) Synchronous bilateral solid papillary carcinomas of the breast. Case Rep Surg 2013: 812129. [Crossref]
- Tariq MU, Idress R, Qureshi MB, Kayani N (2019) Solid papillary carcinoma of breast; a detailed clinicopathological study of 65 cases of an uncommon breast neoplasm with literature review. Breast J 26: 211-215. [Crossref]
