Symptoms Investigate the Frequency of Various Presentations of the Meckel’s Diverticulum and Help Identify Sign Promptly
A B S T R A C T
Background: Meckel's diverticulum is the most common anomaly of the intestine. It is usually asymptomatic but could also be symptomatic with complications such as bleeding, intestinal obstruction, and inflammation. This study was performed to assess the frequency of various presentations of the patients who underwent surgery with the diagnosis of Meckel's diverticulum in Children's Medical Center, Tehran, Iran from March 2005 to March 2011.
Material and Methods: Since this study is a case series report (retrospective descriptive study) and the purpose is assessing the various presentation of Meckel’s` diverticulum, we express the frequency and percent frequency of each presentation. The data collection tool was a five-part survey form. The first part was related to demographic data, the second part was related to clinical data, the third part was related to diagnostic data, the fourth part was related to treatment data and the fifth part was related to histological data. Data were analysed using SPSS statistical program.
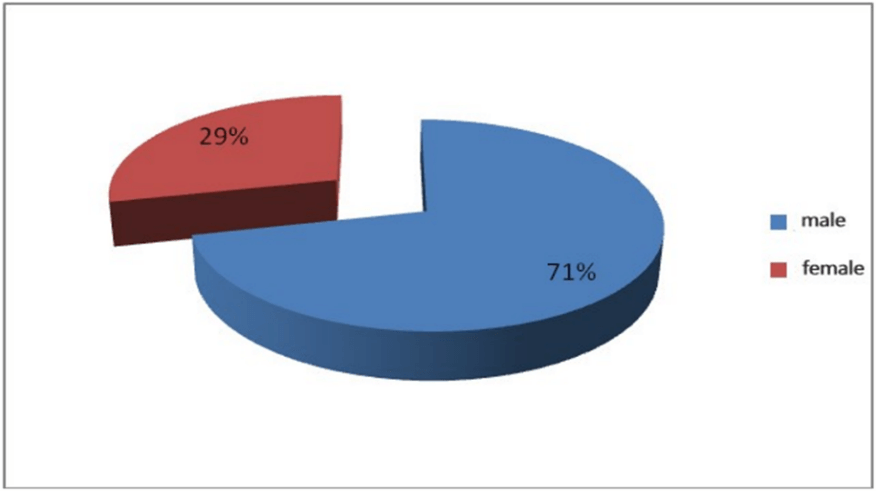
Result: We found 49 patients in this period (71% male 29% female). The mean age was 3.5 years (1 month to 9 years). The male to female ratio was 2.5. The most common clinical symptoms were abdominal pain (63%). forty-three percent of patients had single symptoms and the others had combined symptoms such as abdominal pain and hematochezia. Only 8 patients with lower gastrointestinal bleeding had a Technetium scan and all of them were positive. Associated findings during surgery included appendicitis and invagination. Our sonographic imaging findings were nonspecific. In the pathologic report, 6 patients had gangrene in specimens and 2 perforations. The lining was of gastric type in 24 specimens pancreatic in 3 and mixed in 2 specimens. None of those with the gastric type of mucosa showed Helicobacter pylori infection.
Conclusion: Regarding our findings, clinical findings of Meckel's diverticulum in our study were rather the same as in the literature. Pathologic examination of specimens revealed that most diverticula were lined by gastric type of mucosa. None of those with the gastric type of mucosa showed Helicobacter pylori infection.
Keywords
Presentation, Meckel's diverticulum, Children's Medical Center
Introduction
Meckel’s diverticulum (MD) is the most common congenital abnormality of the gastrointestinal system in pediatric [1-3]. The prevalence of MD in the public population has been estimated at nearly 2%, but retrospective and autopsy studies, have reported range from 0.14% to 4.5% [4]. Approximately 2% of patients develop complications throughout their lives, typically before the age of 2 years [5]. The male to female ratio is 3:1 to 5:1 in older symptomatic children and 1:1 in asymptomatic cases [3]. In Mayo's study on1476 patients, it was reported that the ratio of male/female was 3/1 [6]. Case reports of neonatal MD have been almost exclusively published on male patients [3]. Its occurrence is usually under 10 years of age and the prevalence decreases with increasing age hence, it is rarely found in adults [7]. MD Emerging due to the vitelline duct (VD)'s persistence, which is an embryonic structure that provides relevance from the yolk sac to the midgut during fetal life [3, 7]. MD is almost always placed about 2 feet of the ileocecal valve on the anti-mesenteric border at the terminal ileum and seldom on the mesenteric side due to the embryological origin [5]. Yamakuchi et al. reported an average distance from the ileocecal valve of 50 cm [8].
The rule of Two typically explains its specifications, such as 2% in the general population's rate of prevalence, the ratio of 2:1 in male-to-female, of 2% incidence rate for symptomatic Meckel diverticulum, symptoms' presence in younger than 2 years old cases, 2 feet distance to the ileocecal valve location, 2 inches diverticular length, and 2 common types of ectopic tissues [9]. MD is a kind of true diverticulum, containing all 3 layers of the gastrointestinal wall (mucosa, muscular, and serosa) [2, 7]. The duct generally obliterates during the 7 months of embryonic development, and demolition of closure consequences in a diverticulum 98% of the time [1]. It is usually covered with ileal mucosa-type epithelium but, in about 50% of cases, it comprises of ectopic or heterotopic tissue. Microscopic histopathology discovers the rest of the omphalomesenteric duct comprises heterotopic mucosa in 45%-80% of surgical samples. The most common tissue is gastric mucosa (62 % of cases), but others comprise pancreatic tissue (6%), combined gastric mucosa and pancreatic tissue (5%), jejunal mucosa (2%), Brunner’s glands (2%), and other tissues (10%). which can be the reason for complications [1, 10, 11]. Additionally, MD has a higher chance of becoming symptomatic if the ectopic tissue contains gastric mucosa [3].
The complications of MD are comprised of haemorrhage, perforation, enterolith formation, Littre’s hernia, ulceration, neoplasm, and torsion. Torsion is one of the infrequently reported complications of MD [7]. MD may remain asymptomatic or mimic disorders such as Crohn’s disease, appendicitis, and peptic ulcer disease, so it is difficult to diagnose asymptomatic MD before the operation. The procedure of diagnosing Meckel's diverticulum depends on its clinical symptoms [7, 11]. Diagnostic methods include abdominal ultrasound, abdominal CT scan, upper mesenteric artery angiography, radionuclide scan, and diagnostic laparoscopy [2]. Mortality and disability due to Meckel’s diverticulum are estimated at 12%. Furthermore, the risks of complications in subsequent surgery are estimated at 7% significantly reduced (2%-1%) [12]. Considering the relative frequency of Meckel's diverticulum (2% of the total population) and its diagnostic and treatment problems, and also its complications, we decided to study the frequency of Meckel's diverticula presentations and its complications during the years 2005-2011 in Children’s Medical Center. To approach patients easier when referring to the emergency department and to improve the diagnosis and improve the prognosis of patients.
Methods
This study is a retrospective descriptive study and case series report, and the study population consisted of children undergoing surgery at the Children’s Medical Center Hospital (the scientific center of pediatrics in the country) due to Meckel's diverticulum during the 6 years. First, the names and file numbers of patients were searched from the Pathology Office to collect data. Then by referring to the medical records section, the data were extracted from the files and entered in the data entry sheet (questionnaire). The data collection tool was a five-part survey form (checklist). The first part was related to demographic data, the second part was related to clinical data, the third part was related to diagnostic data, the fourth part was related to therapeutic data and the fifth part was related to histological data. Data were analysed using SPSS statistical program.
Results
Following the review of all available files, 49 identified. The sex distribution of hospitalized patients included 35 male patients (71.4%) and 14 female patients (28.6%) with a ratio of 2.5 to 1. Figure 1 shows the sex relative gender frequency of our patients.
Figure 1: Sex relative gender frequency of understudy patients.
The mean age of patients was 3.5 years, and the age range of cases was 1 month to 9 years. Among patients, 42.85% (21 cases) presented with one clinical symptom, and the rest of the patients presented with 2 or more clinical symptoms. Symptoms in order of prevalence include abdominal pain (63.3%), vomiting (61.2%), bleeding (53.1%), restlessness (42.9%), no defecation (20.40%), and no flatulence (12.2%). Frequency of patients' symptoms at the time of admission are shown in (Table 1). In clinical examination, the most common findings were abdominal tenderness and the presence of blood in rectal examination in 28 patients (57.1%), and the lowest findings related to dehydration in 3 patients (6.1%). The frequency of clinical findings is shown in (Table 2) in order of prevalence. Also, in (Table 3), statistical information related to the level of white blood cells, hemoglobin, and platelets of patients is recorded.
Table 1: Frequency of patients' symptoms at the time of admission.
|
Relative frequency |
absolute frequency |
Clinical sign/symptoms |
|
63.26% |
31 |
Abdominal pain |
|
61.22% |
30 |
Vomiting |
|
48.97% |
24 |
Hematochezia |
|
42.85 % |
21 |
Restlessness |
|
20.40% |
10 |
No defecation |
|
12.24% |
6 |
No gas excretion |
Table 2: Frequency of clinical findings in the examination of patients under study.
|
Relative frequency |
Absolute frequency |
Clinical findings |
|
57.14% |
28 |
Abdominal tenderness |
|
57.14% |
28 |
presence of blood in the RE |
|
28.57% |
14 |
Abdominal distension |
|
14.28% |
7 |
Guarding |
|
12.24% |
6 |
Rebound tenderness |
|
6.12% |
3 |
Dehydration |
Table 3: Statistical indicators of cell count in understudy patients.
|
Indicator |
Minimum |
Maximum |
Average |
Standard deviation |
|
White blood cell*1000 |
6 |
42 |
13/275 |
1601/7 |
|
hemoglobin |
5/5 |
8/16 |
10/851 |
1367/2 |
|
Platelets |
125000 |
666000 |
356000 |
496/108731 |
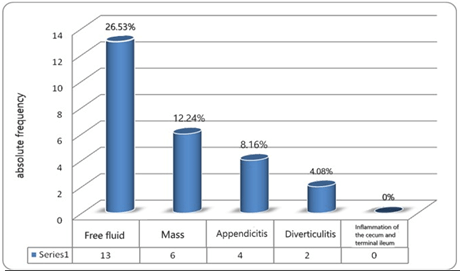
In total, out of 49 cases, 45 patients had ultrasound data. In 25 patients (55.6%) the ultrasound result was normal, and no specific finding was found in favor of Meckel's diverticulum. The most findings in performed ultrasounds were related to the presence of free fluid in 13 patients (26.5%) and the fewest findings were related to inflammation of the cecum and terminal ileum, which was not reported in any cases. The prevalence of different types of ultrasound findings is shown in (Figure 2).
Figure 2: Frequency of various findings in ultrasound.
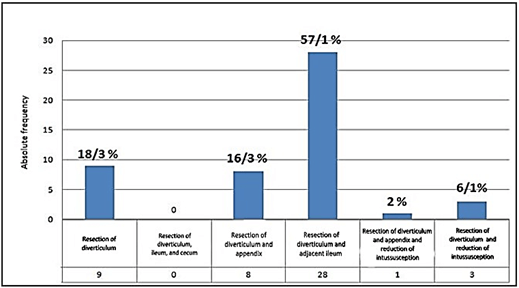
By studying the description of the operation in the patients' files, the results of the frequency of the type of surgery performed in the order of prevalence included the following: Resection of diverticulum and adjacent ileum in 28 patients (57.1%), diverticulum resection in 9 patients (18.3%), resection of the diverticulum and appendix in 8 patients (16.3%), diverticulum resection and intussusception reduction in 3 patients (6.1%), and diverticulum resection and reduction of intussusception and appendectomy in 1 patient (2%); but resection of the diverticulum, cecum and ileum were not reported in any cases. Absolute and relative frequency of surgery in understudy patients are shown in (Figure 3).
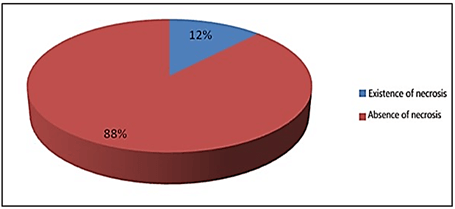
Accompanying findings during diverticulectomy included 8 cases of appendicitis, 4 cases of intussusception, 1 case of volvulus, and 1 case of bilateral inguinal hernia, which included a total of 14 cases (28.5%) of the total cases. Of the appendicitis cases, 4 cases were not diagnosed by ultrasound. In the pathology samples report in the patients' files, necrosis was observed in 6 cases (12.2%). Relative frequency of necrotic tissue in are (Figure 4). Ectopic tissue was present in 29 samples (59.18%), of which 24 samples contained gastric lining (82.75%), 3 samples had pancreatic lining (10.34%) and 2 samples had mixed ectopic tissue lining, relative frequency of ectopic tissue in pathology samples are shown In the (Figure 5) (Were 6.89%) these information are shown (Figure 6). In the study, the frequency of different presentations of Meckel's diverticulum in order of prevalence included: 24 patients (48.97%) gastrointestinal bleeding, 10 patients (20.40%) intestinal obstruction, 8 patients (16.32%) diverticulitis, 4 patients (8.16%) intussusception, 2 case (4.08%) was a perforation, and in 1 case (2.04%) was fistula to the abdominal wall. The frequency of different presentations of Meckel's diverticulum are shown in (Figure 7). Eight patients had a technetium-99 scan, which was positive in all cases.
Figure 3: Absolute and relative frequency of surgery in understudy patients.
Figure 4: Relative frequency of necrotic tissue in pathology specimens.
Figure 5: Relative frequency of ectopic tissue in pathology samples.
Figure 6: Relative frequency of prevalence of ectopic mucosal tissues in pathology samples of understudy patients.
Figure 7: Frequency of different presentations of Meckel's diverticulum.
Discussion
Meckel's diverticulum is a congenital malformation of the gastrointestinal (GI) tract prevalent in roughly 2% of the population, which are often found in children and less present in adults [13, 14]. A large proportion of MD cases in children occur before the age of 2 years, and also There is a 3:2 male to- female prevalence ratio [5, 15]. Even though there is no familial predisposition for MD, but the prevalence is raised in pediatric with other serious diseases such as intestinal obstruction neoplasm, gastroschisis, omphalocele, or malrotation [5]. However most patients with MD are asymptomatic and are incidentally discovered intraoperatively, it can give way to different clinical presentations and complications, and it typically carries a 4-6% lifetime risk of complications including bowel obstruction and intussusception (50.2%), diverticulitis (12.7%), haemorrhage (11.8%), perforation (7.3%), and neoplasm (3.2%) [5, 13, 14]. Searching in the Pediatric Hospital Information System Database for children with symptomatic Meckel’s, Alemayehu et al. found 60.1% of children presenting with obstruction, 35.6% presenting with GI-haemorrhage, and 8.4% presenting with inflammation [16].
In our study, the prevalence of obstruction, gastrointestinal bleeding, and diverticulitis were 20.40%, 48.97%, and 16.32% respectively. In most studies, the symptoms of small bowel obstruction were the most common presentation of MD but in our study gastrointestinal bleeding (49%) was the most common presentation. This may be because our study was performed in the age group of under 9 years, in which bleeding is more common. The clinical presentations of MD differed by gender and age [6, 9, 17]. Symptomatic MD is predominant in males, with a sex ratio ranging from 2:1 to 4:1 [15]. Çelebi et al. assume that the incidence of MD in males is increased as a result of higher gastrin and acid levels in males affect the ectopic gastric mucosa which leads to an increase in MD symptoms [3]. Generally, the complications are seen more often in neonates, infants, and young children compared to adults and more than 50% of patients who develop complications are under 10 years of age [15, 17-19]. Alemayehu and Ruscher found that half of the children operated on for a complication of MD are under 5 years old [16, 20].
The risk of complications decreases with increasing age [18]. For instance, intestinal haemorrhage occurred more frequently in the pediatric population (27%-56%) than in adults (8%-38%) [17-19]. The highest prevalence of obstruction occurs one in infancy and another during 2-5 years of age [6, 21, 22]. It is also reported that idiopathic intussusceptions typically occur between the ages of 3 months and 3 years [9]. Tseng et al. stated that 50% of MD related to intussusception occurs in patients older than 3 years of age [23]. In most studies, the average age of children was 3.5 years, which in our study was 3.5 years too. The average age of adults is in the fourth decade [24]. In Huang et al.’s study, 100 symptomatic Meckel’s diverticula were identified in the age range of 1 day to 18 years old. 17% of them were in the intussusception group with a mean age of 4.55, 24% in the bowel obstruction group with a mean age of 6.6, 44% in the gastrointestinal bleeding group with a mean age of 5.30, and 15% in the diverticulitis and/or perforation group with a mean age of 4.88. The average age of patients in the bowel obstruction group was older than (6.16- 5.80 years). In every group, males accounted for more cases than females [9].
In our study, the mean age of patients with gastrointestinal bleeding presentation (hematochezia, melena) was 3.6 years, obstruction 3.7, diverticulitis 3.2, and perforation 7.5 years. Compared to the study, the mean age of perforation was higher than other common presentation such as bleeding and obstruction. the comparison of the mean age of the most common presentation of MD in our study and the study of Huang et al. are shown in (Table 4). Different presentations of MD have different causes. Simple obstruction due to fibrous bands, volvulus, and internal herniation may be due to the basal anatomy of the diverticulum. Ectopic gastric tissue and colonization by Helicobacter pylori can explain complications such as ulcers, inflammation, obstruction, and bleeding. In the pediatric population, MD is the most common cause of massive lower gastrointestinal (LGI) bleeding, and it is due to the persistence of the proximal part of the congenital vitello-intestinal duct. which is characterized by painless, massive bleeding, and can lead to shock [9, 18]. As 67% to 71% of patients presenting with massive LGI bleeding required blood transfusion [9].
Table 4: Mean age of different presentation of Meckel’s diverticulum.
|
Perforation |
Diverticulitis |
Obstruction |
Gastrointestinal bleeding |
|
|
4.88 |
4.88 |
6.6 |
5.3 |
Huang et al. |
|
7.5 |
3.2 |
3.7 |
3.6 |
Our study |
Complicated MD represents a significant cause of morbidity in children, and it often results from ectopic tissues or bands [17, 18]. Chen et al. noted that the main cause of complicated diverticulum is heterotopic tissue and suggested when it is incidentally discovered in pediatric patients, it should be removed [1]. MD containing gastric heterotopia is present in approximately half of the overall MD cases and up to 80% of symptomatic cases [19]. Microscopic histopathology manifests the heterotopic mucosa in 45%-80% of surgical specimens, mostly gastric mucosa, and sometimes colonic mucosa or pancreatic tissues [1]. Keese et al. reported Heterotopic tissue was confirmed on histopathology in 53% of all patients enclosing gastric, pancreatic, and both gastric and pancreatic mucosae and large intestine tissue could be found, in one case [5]. Rutherford and Akers studied 147 surgical specimens and found heterotopic tissue in 57% [25, 26]. Burjonrappa and Khaing stated that the existence of ectopic tissue is the most important factor which in patients with MD specifying the need for surgical intervention [27].
In our study, ectopic tissue was present in 29 patients (59.18%), of which 24 cases contained gastric lining (82.75%), 3 cases had pancreatic lining (10.34%) and 2 cases had mixed ectopic tissue lining (were 6.89%). Unfortunately, MD has varied clinical appearance for different individuals like painless lower gastrointestinal bleeding, abdominal distention, recurrent vague abdominal pain, nausea, and vomiting. These nonspecific presentations are usually confused with those of appendicitis, inflammatory bowel disease, and other clinical scenarios [1]. Accurate preoperative diagnosis of MD is notably difficult in symptomatic patients as the clinical presentation is nonspecific and mimics many other abdominal diseases and can be easily misdiagnosed which is why pediatricians and pediatric surgeons should be well aware of its feasible presentations. This is especially true in patients who represent symptoms other than bleeding [5, 14, 17]. Charki et al. found that complicated MD must be one of the diagnoses to be evoked in front of an acute abdomen, intestinal obstruction, or low digestive haemorrhage [28].
Kusumoto et al. in a study of 776 patients have reported in 88% of patients represent bleeding had a correct, preoperative diagnosis against 11% with symptoms other than bleeding [29]. Several imaging modalities, containing ultrasound, plain film, and CT, are challenging as it shows non-specific changes and is difficult to distinguish between MD and a loop of bowel, thus restricting its detection [14]. Radiography helps diagnose intestinal obstruction and can show air or fluid-air levels in the diverticulum. Barium examination also rarely detects Meckel's diverticulum (small diverticulum opening) [23, 30]. Srisajjakul et al. found that the radiological diagnosis is of paramount importance for proper patient management [10]. Dalinka and Wunder (1973) found radiological abnormalities in 10-17 patients and only 3 patients were the diverticulum demonstrated radiologically [31]. Ultrasound can help diagnose a diverticulum when it has complications. In cases where the diverticulum is obstructed (fluid-filled diverticulum), it is seen as a dilated tubule (fluid-filled diverticulum).
Also, in our study, the sonographic findings were nonspecific. The highest findings were related to the presence of free fluid (26.53 %), respectively: mass (12.24 %), appendicitis (8.16 %), and diverticulitis in 2 patients (4.08%). Ultrasound results were normally reported in 25 patients (51.02%) due to a lack of patient cooperation or inaccuracy of the sonographer. If the Meckel's diverticulum shows painless rectal bleeding, the most sensitive diagnostic test is a radionuclide scan performed after the injection of technetium 99 pertechnetate as heterotopic gastric mucosa is present, and 99m-technetium pertechnetate is taken up by the heterotopic gastric mucosa in MD, so 99m-technetium pertechnetate scintigraphy can provide an important clue to diagnosing MD. the sensitivity of scintigraphy is 85% to 54%, and it is highly specific (95%) [14]. In 1967, Harden et al. demonstrated that technetium 99m was concentrated in the gastric mucosa [26]. It was reported that certain substances, such as pentagastrin, histamine-2 blockers, and glucagons, would increase the diagnostic yield of the Meckel's scan [23, 30]. In all the mentioned methods, the findings are nonspecific.
In our study, based on patient records, radionuclide scans were requested for 8 patients who were diagnosed with Meckel's diverticulum. The above 8 patients presented with gastrointestinal bleeding and ectopic gastric mucosal pathology was reported. Delayed diagnosis may cause extremely serious complications, such as intestinal gangrene and perforation, peritonitis, and sepsis, and may even result in life-threatening consequences [1]. In our study, 6 cases of necrosis were observed (12.24%). There has been an ongoing argument about the excision of Meckel’s diverticulum when found as an asymptomatic accidental finding [18]. Although it is widely accepted that symptomatic MD should be resected, the management of MD incidentally detected during abdominal surgery is still controversial [19]. It is generally impossible, during operation to specify by detection or palpation whether incidentally found Meckel’s diverticulum is at raised risk of the complications or not [18].
Park et al. favored removal of incidental asymptomatic Meckel’s diverticulum in males, patients younger than 50 years, diverticulum greater than 2 cm, and the presence of histological abnormal tissue [6]. Cullen et al. held that prophylactic resection is recommended except in the face of contraindications like generalized peritonitis or other conditions that make resection more hazardous and also found that the risk of developing symptomatic Meckel’s did not decrease with age [32]. Gezer et al. considered that incidentally detected MD should be removed regardless of its macroscopic appearance [33]. Tauro et al. suggest resection of a normal-appearing MD in every case of appendectomy or laparotomy/laparoscopy for an acute abdomen to avoid secondary complications arising from it [34]. However, Soltero and Bill did not favor incidental diverticulectomy, because they noted a 4.2% lifetime complication risk of Meckel’s diverticulum against 9% morbidity after incidental resection [35]. Adversaries of routine resection of asymptomatic Meckel’s diverticulum held the morbidity and mortality of resection are greater than the computed hazardous of developing complications [36]. A study on 202 cases manifested that after prophylactic diverticulectomy the risk of postoperative complications was much higher than the risk of complications relevant to the MD itself [25].
It is reported in various surgical series that the average mortality from Meckel’s diverticulum is around 6%, with a large proportion of deaths occurring in aged people [11]. Cullen et al. reported morbidity of 12% for resection of symptomatic MD in adults, and the cumulative risk of long-term postoperative complications was 7% [32]. In a systematic review by Zani et al., the postoperative morbidity was 5.3%, with wound infections being the most common complication [37]. Nevertheless, the treatment of choice for the symptomatic Meckel’s diverticulum is surgical resection. and this can be achieved either by the diverticulectomy or by the segmental bowel resection and anastomosis, especially when there is palpable ectopic tissue at the diverticular-intestinal junction, perforation, or intestinal ischaemia [19]. The choice of surgical techniques depends on the external appearance of the MD. Also, a length-to-diameter ratio is helpful to determine the surgical approach [9]. Chan et al. reported that for the management of complicated Meckel’s diverticulum, Laparoscopy is a safe and effective method [38]. In our study, the highest frequency of surgery was related to the resection of the diverticulum and adjacent ileum. However, hypertrophic gastric mucosa with colonization by Helicobacter pylori increases the incidence of complications such as obstruction, inflammation, and perforation [39]. In our study, Helicobacter pylori were not found in any of the gastric lining specimens.
Conclusion
The findings of this study are similar to other studies in terms of age and sex. In terms of the frequency of various presentations of the Meckel's diverticulum, our most common finding was lower gastrointestinal bleeding followed by obstruction. Among the preoperative diagnostic methods for diagnosing MD complications such as lower gastrointestinal bleeding, radionuclide scanning is the best diagnostic tool and ultrasound helps diagnose complications such as obstruction and is not diagnostic in cases of diverticulitis.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 29, Oct 2020Accepted: Mon 09, Nov 2020
Published: Thu 26, Nov 2020
Copyright
© 2023 Ashjaei Bahar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GSCR.2020.02.06
Author Info
Ashjaei Bahar Amiri Shakiba Najdi Fatemeh Movahedi Jadid Merisa
Corresponding Author
Ashjaei BaharDepartment of Pediatric Surgery, Children Medical Center of Excellence, Tehran University of Medical Sciences, Tehran, Iran
Figures & Tables
Table 1: Frequency of patients' symptoms at the time of admission.
|
Relative frequency |
absolute frequency |
Clinical sign/symptoms |
|
63.26% |
31 |
Abdominal pain |
|
61.22% |
30 |
Vomiting |
|
48.97% |
24 |
Hematochezia |
|
42.85 % |
21 |
Restlessness |
|
20.40% |
10 |
No defecation |
|
12.24% |
6 |
No gas excretion |
Table 2: Frequency of clinical findings in the examination of patients under study.
|
Relative frequency |
Absolute frequency |
Clinical findings |
|
57.14% |
28 |
Abdominal tenderness |
|
57.14% |
28 |
presence of blood in the RE |
|
28.57% |
14 |
Abdominal distension |
|
14.28% |
7 |
Guarding |
|
12.24% |
6 |
Rebound tenderness |
|
6.12% |
3 |
Dehydration |
Table 3: Statistical indicators of cell count in understudy patients.
|
Indicator |
Minimum |
Maximum |
Average |
Standard deviation |
|
White blood cell*1000 |
6 |
42 |
13/275 |
1601/7 |
|
hemoglobin |
5/5 |
8/16 |
10/851 |
1367/2 |
|
Platelets |
125000 |
666000 |
356000 |
496/108731 |
Table 4: Mean age of different presentation of Meckel’s diverticulum.
|
Perforation |
Diverticulitis |
Obstruction |
Gastrointestinal bleeding |
|
|
4.88 |
4.88 |
6.6 |
5.3 |
Huang et al. |
|
7.5 |
3.2 |
3.7 |
3.6 |
Our study |



References
- Chen Q, Gao Z, Zhang L, Zhang Y, Pan T et al. (2018) Multifaceted behavior of Meckel's diverticulum in children. J Pediatr Surg 53: 676-681. [Crossref]
- Erol V, Yoldaş T, Cin S, Çalışkan C, Akgün E et al. (2013) Complicated Meckel's diverticulum and therapeutic management. Ulus Cerrahi Derg 29: 63-66. [Crossref]
- Çelebi S (2017) Male predominance in Meckel's diverticulum: A hyperacidity hypotheses. Med Hypotheses 104: 54-57. [Crossref]
- Lin XK, Huang XZ, Bao XZ, Zheng N, Xia QZ et al. (2017) Clinical characteristics of Meckel diverticulum in children: A retrospective review of a 15-year single-center experience. Medicine 96: e7760. [Crossref]
- Keese D, Rolle U, Gfroerer S, Fiegel H (2019) Symptomatic Meckel's Diverticulum in Pediatric Patients-Case Reports and Systematic Review of the Literature. Front Pediatr 7: 267. [Crossref]
- Park JJ, Wolff BG, Tollefson MK, Walsh EE, Larson DR (2005) Meckel diverticulum: the Mayo Clinic experience with 1476 patients. Ann Surg 241: 529-533. [Crossref]
- Ajmal HB, Majid Z, Tahir F, Sagheer S (2020) Axial Torsion and Gangrene: An Unusual Complication of Meckel's Diverticulum. Cureus 12: e6702. [Crossref]
- Ymaguchi M, Takeuchi S, Awazu S (1978) Meckel’s diverticulum: investigation of 600 patients in Japanese literature. Am J Surg 136: 247-249. [Crossref]
- Huang CC, Lai MW, Hwang FM, Yeh YC, Chen SY et al. (2014) Diverse presentations in pediatric Meckel's diverticulum: a review of 100 cases. Pediatr Neonatol 55: 369-375. [Crossref]
- Srisajjakul S, Prapaisilp P, Bangchokdee S (2016) Many faces of Meckel's diverticulum and its complications. Jpn J Radiol 34: 313-320. [Crossref]
- Malik AA, Bari S, Wani KA, Khaja AR (2010) Meckel's diverticulum-Revisited. Saudi J Gastroenterol 16: 3-7. [Crossref]
- Silber G (1990) Lower gastrointestinal bleeding. Pediatr Rev 12: 85-93. [Crossref]
- Spangler H, Fisher J (2020) The rule of two's didn't work: Meckel's diverticulum with hemorrhagic shock in an adolescent. Am J Emerg Med 38: 1541.e1-1541.e2. [Crossref]
- Zhu Y, Dong M, Weng W, Yang J (2018) Spontaneous perforation and intraabdominal abscess due to Meckel's diverticulum revealed on SPECT/CT with 99m-technetium pertechnetate: A case report. Medicine 97: e13004. [Crossref]
- Parvanescu A, Bruzzi M, Voron T, Tilly C, Zinzindohoué F et al. (2018) Complicated Meckel's diverticulum: Presentation modes in adults. Medicine 97: e12457. [Crossref]
- Alemayehu H, Hall M, Desai AA, Peter SD, Snyder CL (2014) Demographic disparities of children presenting with symptomatic Meckel’s diverticulum in children’s hospitals. Pediatr Surg Int 30: 649-653. [Crossref]
- Chen JJ, Lee HC, Yeung CY, Chan WT, Jiang CB et al. (2014) Meckel's Diverticulum: Factors Associated with Clinical Manifestations. ISRN Gastroenterol 2014: 390869. [Crossref]
- Sagar J, Kumar V, Shah DK (2006) Meckel's diverticulum: a systematic review. J R Soc Med 99: 501-505. [Crossref]
- Slívová I, Vávrová Z, Tomášková H, Okantey O, Penka I et al. (2018) Meckel's Diverticulum in Children-Parameters Predicting the Presence of Gastric Heterotopia. World J Surg 42: 3779-3784. [Crossref]
- Ruscher KA, Fisher JN, Hughes CD, Neff S, Lerer TJ et al. (2011) National trends in the surgical management of Meckel's diverticulum. J Pediatr Surg 46: 893-896. [Crossref]
- Schmid SW, Schafer M, Krachenbuhl L, Buchler MW (1999) The role of Laparoscopy in symptomatic Meckel`s Diverticulum. Surg Endosc 13: 1047-1049. [Crossref]
- Spencer R (1964) Gastrointestinal Hemorrhage in infancy and childhood: 476 cases. Surgery 55: 718-734. [Crossref]
- Tseng YY, Yang YJ (2009) Clinical and diagnostic relevance of Meckel’s diverticulum in children. Eur J Pediatr 168: 1519-1523. [Crossref]
- Passarge E, Stevenson RE (2006) Meckel`s Diverticulum. In: Human Malformation and Related anomalies, 2nd ed, Stevenson RE, Hall JE (Eds). Oxford University Press 2006: 1111.
- Heldans F Cited by Neff G (1937) Das Maxkelsche Divertikel Erbgeb. Chir Orthop 30: 227-315.
- Harden R, Alexander WD (1967) Isotope uptake and scanning of Stomach in man with 99mTc-pertechnetate. Lancet 1: 1305-1307. [Crossref]
- Burjonrappa S, Khaing P (2014) Meckel’s diverticulum and ectopic epithelium: evaluation of a complex relationship. J Indian Assoc Pediatr Surg 19: 85-89. [Crossref]
- Charki MT, Oukhouya MA, Benmassaoud Z, Mahmoudi A, Khattala K et al. (2019) Complications of Meckel's diverticulum in children: about 18 cases. Pan Afr Med J 33: 113. [Crossref]
- Kusumoto H, Yoshida M, Takahashi I, Anai H, Maehara Y et al. (1992) Complications and diagnosis of Meckel’s diverticulum in 776 patients. Am J Surg 164: 382-383. [Crossref]
- Rho JH, Kim JS, Kim SY, Kim SK, Choi YM et al. (2013) Clinical features of symptomatic Meckel's diverticulum in children: comparison of scintigraphic and non-scintigraphic diagnosis. Pediatr Gastroenterol Hepatol Nutr 16: 41-48. [Crossref]
- Levator JH Cited by Curd HH (1923) A histological study of Meckel’s diverticulum with special reference to heterotopic tissues. Arch Surg 12: 5069-5523.
- Cullen JJ, Kelly KA, Moir CR, Hodge DO, Zinsmeister AR et al. (1994) Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg 220: 564-568. [Crossref]
- Gezer HÖ, Temiz A, İnce E, Ezer SS, Hasbay B et al. (2016) Meckel diverticulum in children: Evaluation of macroscopic appearance for guidance in subsequent surgery. J Pediatr Surg 51: 1177-1180. [Crossref]
- Tauro LF, Martis JJ, Menezes LT, Shenoy HD (2011) Clinical profile and surgical outcome of Meckel’s diverticulum. J Indian Med Assoc 109: 489-490. [Crossref]
- Soltero MJ, Bill AH (1976) The natural history of Meckel’s diverticulum and its relation to incidental removal. A study of 202 cases of diseased Meckel’s Diverticulum found in King County, Washington, over a fifteen-year period. Am J Surg 132: 168-173. [Crossref]
- McKay R (2007) High incidence of symptomatic Meckel's diverticulum in patients less than fifty years of age: an indication for resection. Am Surg 73: 271-275. [Crossref]
- Zani A, Eaton S, Rees CM, Pierro A (2008) Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg 247: 276-281. [Crossref]
- Chan KW, Lee KH, Mou JWC, Cheung ST, Tam YH (2008) Laparoscopic management of complicated Meckel's diverticulum in children: a 10-year review. Surg Endosc 22: 1509-1512. [Crossref]
- Oguzkurt P, Talim B, Tanyel FC, Caglar M, Senocak ME et al. (2001) The role of heterotopic gastric mucosa with or without colonization of Helicobacter pylori upon the diverse symptomatology of Meckel's Diverticulum in Children. Turk J Pediatr 43: 312-316. [Crossref]
