Journals
Surgical Removal of Inadvertent Portal Thrombosis by Rescue-ALPPS for Perihilar Cholangiocarcinoma
A B S T R A C T
Introduction:Treatment of perihilar cholangiocarcinoma (PHC) usually requires extended resection after inducing hypertrophy of the future liver remnant (FLR). Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) can achieve rapid hypertrophy of the FLR. Though, due to significant morbidity and mortality, portal vein embolization (PVE) is considered gold standard. Despite remaining controversies, ALPPS might suit as reserve in patients who failed to achieve adequate hypertrophy of the FLR or suffered complications following PVE. We illustrate a rescue-ALPPS after inadvertent nontarget thrombosis of the FLR following PVE in a patient with PHC. Presentation of Case:A 67-year-old patient requiring right trisectionectomy for PHC Bismuth type IV suffered inadvertent nontarget portal thrombosis of the FLR following PVE. Subsequently, insufficient FLR hypertrophy prevented the planned surgical resection. ALPPS procedure with concomitant thrombectomy of the left portal vein was used as a rescue strategy for this patient. Discussion:Since ALPPS is associated with significant limitations, especially in patients with PHC, this approach remains controversial. However, surgery still remains the only curative option for patients with PHC and thus, in case of inadequate hypertrophy of the FLR or technical failure following PVE, these patients lack further treatment options. Recent technical refinements and methods of improved patient selection have the potential to emend outcomes of ALPPS in experienced centres. Conclusion:ALPPS should be considered as reasonable rescue strategy not only in case of insufficient hypertrophy of the FLR but also in the event of technical failure or complications following PVE, even in patients with PHC.
Keywords
Case report,rescue-ALPPS,portal vein embolization,perihilar cholangiocarcinoma
Introduction
Regarding treatment of perihilar cholangiocarcinoma (PHC), surgery still represents the only curative option. Usually extended hepatectomy after inducing hypertrophy of the future liver remnant (FLR) is required [1]. Insufficient FLR is associated with a higher rate of post-hepatectomy liver failure (PHLF), which also significantly affects mortality [2, 3]. Hence, for patients undergoing major hepatic resection a minimum remnant liver volume to total liver volume ratio (RLV-TLV) of 30% respectively remnant liver volume to body weight ratio (RLV-BWR) of 0.5% is recommended to avoid PHLF [4]. Preoperative portal vein embolization (PVE) is considered the gold standard to mediate hypertrophy of the FLR and thereby reduce the risk of developing PHLF [5]. In case of inadvertent non-target thrombosis of the left portal vein during embolization, few treatment options remain for the patient aiming for right trisectionectomy [6]. We hereby present a case of surgical removal of inadvertent nontarget thrombosis of the FLR following PVE in terms of rescue-ALPPS in a patient with PHC in accordance with the SCARE and PROCESS criteria [7, 8].
ALPPS was introduced in 2012 as a new technique, which can achieve enhanced hypertrophy of the FLR prior to extended hepatic resection and thereby improves resectability [9]. Since ALPPS is associated with significant morbidity and mortality, especially in patients with PHC, this approach remains controversial [10-13]. The current case report provides a surgical solution treating PVE complications as well as enhancing FLR hypertrophy by in-situ splitting of the liver.
Presentation of Case
We report the case of a 67-year-old patient who initially presented with jaundice and pain in the right upper quadrant of the abdomen to a secondary care centre. He denied nausea, fever, weight loss and night sweat. Endoscopic retrograde cholangiography (ERCP) revealed a stenosis of the common hepatic duct extending a few millimetres into all branches of the biliary trifurcation. These findings supported the suspected diagnosis of perihilar cholangiocarcinoma Bismuth type IV. Brush cytology, however, showed no evidence of malignancy. Subsequently a pigtail stent was placed into the left hepatic duct (double pigtail endoprosthesis 8.5-French). Concerning the right hepatic duct, stenting remained unsuccessful due to configuration of the stenosis. After referral to our surgical department, computer tomography (CT) imaging provided no signs of distant metastases. First, with resolving cholestasis the patient was scheduled for open surgical exploration to establish definitive diagnosis and to evaluate the possibility of right trisectionectomy. Intraoperatively the suspected tumour appeared with a distinct right-sided predominance (segments IV, V to VIII). Histopathology confirmed the diagnosis of adenocarcinoma.
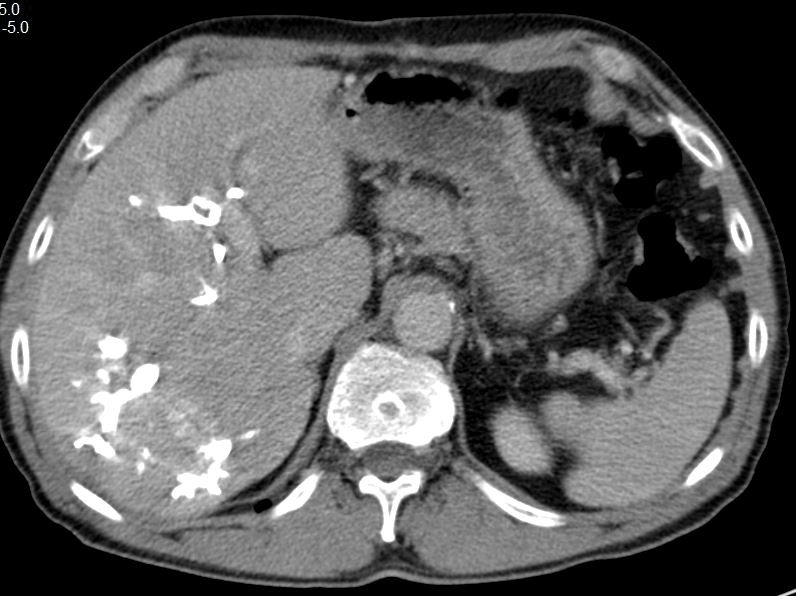
Figure 1: Initial CT volumetry of left-lateral liver segments II and III yielded 161 ml (RLV-BWR was 0.22%) prior to PVE
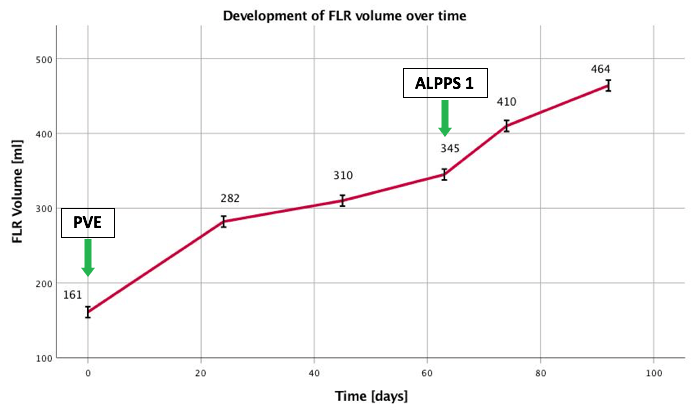
In view of the present findings and the insufficient volume of the FLR, it was decided to initially aim for augmentation by means of right portal vein embolization prior to right trisectionectomy. CT volumetry of left-lateral liver segments II and III yielded 161 ml (Figure 1). Taking into account the body weight of 72 kg, the RLV-BWR was 0.22% at this time. Due to the low volume of the FLR, a high risk of PHLF was assumed. In order to achieve the minimum RLV-BWR of at least 0.5%, a target volume of 360 ml was required. Subsequently, selective embolization of portal vein branches supplying liver segments IV to VIII succeeds using a histoacryl/lipiodol mixture at a 2:1 ratio. Postinterventional angiographic control indicates occlusion of the right portal vein, including segment IV branches and patent left portal vein. CT follow-up after 24 days showed a FLR volume of 282 ml (RLV-BWR 0.39%). Further imaging studies still resulted in marginal volume increase of FLR 45 (310ml; RLV-BWR 0.43%) and 63 (345 ml; RLV-BWR 0.48%) days after PVE (Figure 2). Inadvertent nontarget thrombus in the left branch of the portal vein caused by PVE was revealed in the CT scan. Partial occlusion of the left portal vein was supposed to be the reason for insufficient hypertrophy of the left lateral liver lobe even 9 weeks after PVE. We opt for proceeding with ALPPS procedure with concomitant thrombectomy of the left portal vein as a rescue strategy for this patient.
Figure 2: The depicted figure illustrates the development of the FLR volume and the calculated RLV-BWR over time
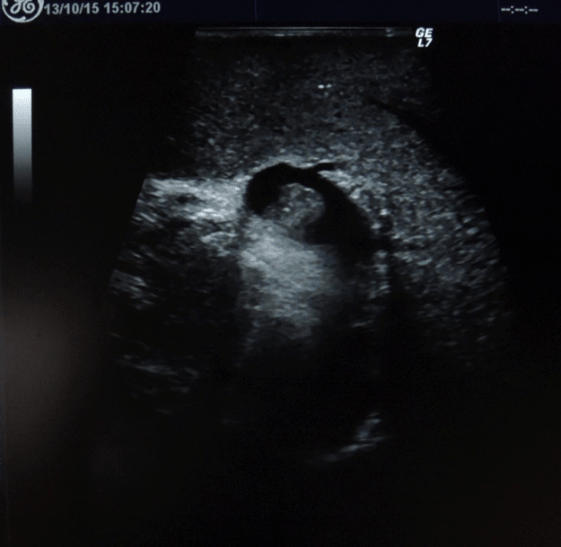
Figure 3: Intraoperative ultrasound imaging of inadvertent nontarget thrombus in the left branch of the portal vein following PVE (indicated by the red arrow)
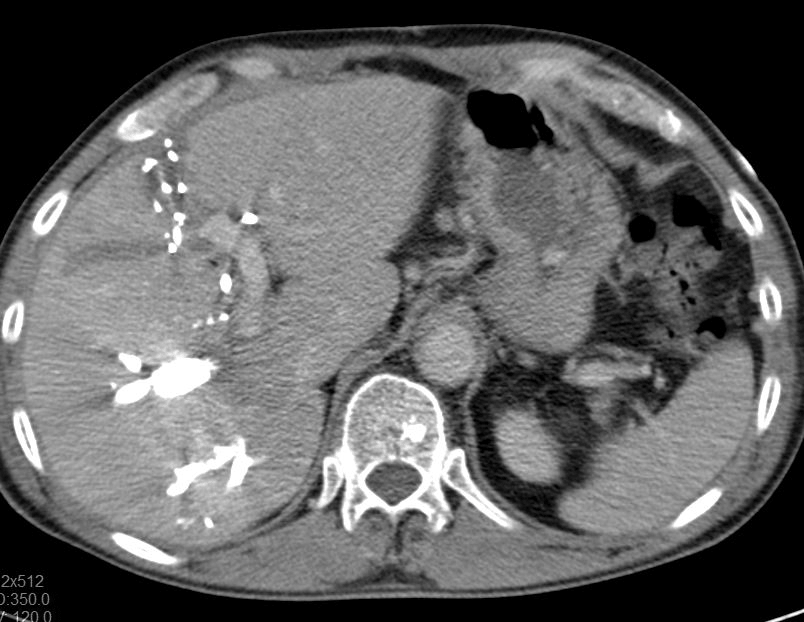
Intraoperatively, the partial embolization of the left portal vein was confirmed by ultrasound examination (Figure 3). Intrahepatic portion of the left portal vein was exposed by partial transection of the liver parenchyma along the falciform ligament (Figure 4). Under inflow occlusion using Pringle manoeuvre, a venotomy was carried out at the ventral side of the intrahepatic left portal vein. Thrombus was completely removed and venotomy was closed with 6-0 prolene sutures. Ultrasound confirmed the patency of the portal vein. Postoperatively, the patient developed angina pectoris pain. Electrocardiogram (ECG) and coronary angiography diagnosed new-onset atrial fibrillation, Takotsubo cardiomyopathy and one-vessel coronary artery disease (left coronary artery, LAD). However, there was no indication for urgent coronary intervention. An antiplatelet therapy and anticoagulation (grade II according to the Clavien-Dindo) was initiated [14]. Further postoperative course was uneventful. Imaging controls showed slow but sufficient volume increase of FLR (464 ml; RLV-BWR 0.64%) after ALPPS stage one on postoperative day 28 (Figure 5). Finally, completion of ALPPS stage two could successfully achieved on postoperative day 34 by means of right trisectionectomy including resection of the extrahepatic biliary tree and portal vein bifurcation as well as regional lymphadenectomy. No PHLF or portal vein thrombosis was observed during further course.
Figure 4: Exposition of the intrahepatic portion of the left portal vein by partial transection of the liver parenchyma along the falciform ligament
Figure 5: CT volumetry after ALPPS stage one shows sufficient volume increase of FLR (464 ml; RLV-BWR 0,64%) on postoperative day 28
Discussion
After initial enthusiasm driven by the perception of expanding resectability by rapid increase of FLR, ALPPS is appraised increasingly controversial, especially in patients with PHC, due to high morbidity and mortality rates in early reported series of ALPPS [10-13, 15]. PVE remains standard of care for treatment of PHC despite a known drop-out rate of 25% [16]. Migration of embolic material into the main portal or left portal vein is one reason for technical failure after PVE [6, 16]. In such a case, efficient treatment options are scarce [6]. The innovation of ALPPS provides a surgical strategy to handle such complications as demonstrated in the current case report. By splitting the liver parenchyma, the intrahepatic portion of the left portal vein was exposed. Under temporary occlusion of the inflow, the intraluminal thrombosis could be removed without pressure. Additionally, liver transection contributed to further hypertrophy of the FLR. Liver transection in combination of right PVE was reported as hybrid-ALPPS [17]. In-situ liver transection after insufficient volume increase post-PVE was also described as rescue-ALPPS [18, 19]. Both methods lead to adequate hypertrophy of the FLR. Superior surgical outcome of ALPPS in comparison to PVE has been confirmed by recent completed randomized trial [20]. Surgical refinement as well as proper patient selection keep on reducing the morbidity and mortality after ALPPS [13, 21-23]. Referring to this matter, diminution of surgical trauma especially regarding technically demanding first stage is considered key of the revised surgical strategy of ALPPS [24].
We presented the case of a Rescue-ALPPS procedure after inadvertent nontarget thrombosis of the future liver remnant following PVE in a patient with PHC. As previously demonstrated, ALPPS is a considerable rescue strategy in patients who failed to achieve adequate hypertrophy following PVE [18, 19]. Regarding the current report, ALPPS should be taken into account also in the event of technical failure or complications following PVE.
Conclusion
ALPPS should be considered as reasonable rescue strategy not only in case of insufficient hypertrophy of the FLR but also in the event of technical failure or complications following PVE. Particularly, regarding the significant technical refinements after overcoming early learning curve and improved patient selection. With regard to significant morbidity and mortality rates still reported in previous studies, this technically challenging approach remains reserved to experienced centres.
Conflicts of Interest
Jens Rolinger and Jun Li have no conflict of interest.
Funding
Jens Rolinger and Jun Li have no study sponsor.
Ethical Approval
Ethical approval was not necessary for this study.
Consent
We obtained written patient consent to publication.
Author Contribution
All authors designed the study, collected data and interpreted data. Jens Rolinger wrote the paper and Jun Li revised.
Guarantor
Jun Li.
Registration of research studies
Not applicable.
List of abbreviations
- ALPPS : Associating liver partition and portal vein ligation
- CT : Computer tomography
- ECG : Electrocardiogram
- ERCP : Endoscopic retrograde cholangiography
- FLR : Future liver remnant
- LAD : Left coronary artery
- PHC : Perihilar cholangiocarcinoma
- PVE : ßPortal vein embolization
- RLV-BWR : Remnant liver volume to body weight ratio
- RLV-TLV : Remnant liver volume to total liver volume ratio
Article Info
Article Type
Case ReportPublication history
Received: Thu 30, May 2019Accepted: Wed 19, Jun 2019
Published: Tue 16, Jul 2019
Copyright
© 2023 Jun Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJSCR.2019.01.06
Author Info
Corresponding Author
Jun LiDepartment of General, Visceral and Thoracic Surgery, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany
Figures & Tables

References
- Nagino M (2012) Perihilar cholangiocarcinoma: a surgeon's viewpoint on current topics. J Gastroenterol 47: 1165-1176. [Crossref]
- Ribero D, Zimmitti G, Aloia TA, Shindoh J, Fabio F et al. (2016) Preoperative Cholangitis and Future Liver Remnant Volume Determine the Risk of Liver Failure in Patients Undergoing Resection for Hilar Cholangiocarcinoma. J Am Coll Surg 223: 87-97. [Crossref]
- Olthof PB, Wiggers JK, Groot Koerkamp B, Coelen RJ, Allen PJ et al. (2017) Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J Am Coll Surg 225: 387-394. [Crossref]
- Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L et al. (2007) Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 204: 22-33. [Crossref]
- Narula N, Aloia TA (2017) Portal vein embolization in extended liver resection. Langenbecks Arch Surg 402: 727-735. [Crossref]
- van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS et al. (2013) Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 36: 25-34. [Crossref]
- Agha RA, Fowler AJ, Saeta A, Barai I, Rajmohan S et al. (2016) The SCARE Statement: Consensus-based surgical case report guidelines. Int J Surg 34: 180-186. [Crossref]
- Agha RA, Fowler AJ, Rajmohan S, Barai I, Orgill DP et al. (2016) Preferred reporting of case series in surgery; the PROCESS guidelines. Int J Surg 36: 319-323. [Crossref]
- Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J et al. (2012) Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 255: 405-414. [Crossref]
- Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M et al. (2017) High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 19: 381-387. [Crossref]
- de Santibanes E, Clavien PA (2012) Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg 255: 415-417. [Crossref]
- Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M et al. (2016) Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 103: 1768-1782. [Crossref]
- Linecker M, Bjornsson B, Stavrou GA, Oldhafer KJ, Lurje G et al. (2017) Risk Adjustment in ALPPS Is Associated with a Dramatic Decrease in Early Mortality and Morbidity. Ann Surg 266: 779-786. [Crossref]
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213. [Crossref]
- Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY et al. (2013) Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 217: 126-133. [Crossref]
- Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y et al. (2012) Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg 29: 23-29. [Crossref]
- Li J, Kantas A, Ittrich H, Koops A, Achilles EG et al. (2016) Avoid "All-Touch" by Hybrid ALPPS to Achieve Oncological Efficacy. Ann Surg 263: e6-e7. [Crossref]
- Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M et al. (2013) In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg 100: 388-394. [Crossref]
- Enne M, Schadde E, Bjornsson B, Hernandez Alejandro R, Steinbruck K et al. (2017) ALPPS as a salvage procedure after insufficient future liver remnant hypertrophy following portal vein occlusion. HPB (Oxford) 19: 1126-1129. [Crossref]
- Sandstrom P, Rosok BI, Sparrelid E, Larsen PN, Larsson AL et al. (2018) ALPPS Improves Resectability Compared with Conventional Two-stage Hepatectomy in Patients with Advanced Colorectal Liver Metastasis: Results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 267: 833-840. [Crossref]
- Petrowsky H, Gyori G, de Oliveira M, Lesurtel M, Clavien PA (2015) Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 261: e90-e92. [Crossref]
- de Santibanes E, Alvarez FA, Ardiles V, Pekolj J, de Santibanes M (2016) Inverting the ALPPS paradigm by minimizing first stage impact: The Mini-ALPPS technique. Langenbecks Arch Surg 401: 557-563. [Crossref]
- Lang H, de Santibanes E, Clavien PA (2017) Outcome of ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 19: 379-380. [Crossref]
- Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V et al. (2015) Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol 41: 674-682. [Crossref]
