Surgical Planning of Hepatic Metastasectomy Using Radiologist-Performed Intraoperative Ultrasound
A B S T R A C T
Background: Intraoperative ultrasound (IOUS) of the liver is a useful adjunct for surgical planning during hepatic metastasectomy. This study aims to (1) report the frequency of change in operative plan as a result of IOUS findings and (2) determine whether IOUS is still beneficial after implementing a standardized, comprehensive process of preoperative hepatic imaging.
Methods: First, a retrospective review of all patients undergoing hepatic metastasectomy at a single institution was conducted to identify how frequently IOUS findings altered the surgical plan. Second, a prospective study was conducted where patients underwent both preoperative CT and MRI within 30 days before surgery to determine if IOUS may still have benefit despite the implementation of a standardized preoperative imaging protocol.
Results: In the retrospective review, 39 liver resections were completed; 100% and 36% of patients underwent preoperative CT and MRI, respectively. The mean time between preoperative imaging and surgery was 46 days (7-126). Operative plans were changed in 10/39 (26%) cases based on IOUS. After the standardization of preoperative imaging, 27 liver resections were performed. All patients underwent preoperative CT and MRI; the mean time between preoperative imaging and surgery was 20 days (1-98) (p=0.001). The operative plan was amended in 5/27 (19%) cases based on IOUS (χ2=1.405, p=0.24).
Conclusion: Even after standardizing the quality and timing of preoperative imaging, the operative plan was changed in nearly 1/5 patients due to IOUS. These findings demonstrate the utility of IOUS in surgical planning for hepatic metastasectomy and provide the basis for a quality improvement strategy regarding standardized preoperative imaging.
Keywords
Liver resection, metastases, ultrasound, imaging, standardization, operative plan
Introduction
Surgical planning for hepatic resection of liver metastases takes place after staging with imaging modalities, such as CT scan and MRI. Several studies have shown that despite advances in preoperative imaging in detecting liver metastases, intraoperative ultrasound (IOUS) of the liver may be superior to preoperative imaging in screening for metastases and planning for curative surgery [1, 2]. Studies have shown that IOUS can detect occult lesions that were missed by preoperative imaging or developed in the time period between preoperative imaging and surgery. Reported IOUS detection rates for occult lesions have varied from 10-47%, leading to changes in surgical strategy in 3-18% of cases [3-6]. Since there are no harms associated with IOUS to the patient, some authors have suggested that IOUS should be routinely used during liver resection of colorectal metastases [1, 2].
However, recent advancements in preoperative imaging modalities, such as the development of new contrast media, have improved preoperative detection of even sub-centimetre hepatic lesions [7, 8]. This has challenged the value of IOUS. Multiple recent studies have found that the sensitivity of multidetector CT and preoperative MRI in detecting liver metastases is equivalent to or higher than that of IOUS [5, 9].
Our institution uses a multidisciplinary team approach between surgeons and interventional radiologists when planning the surgical treatment of liver metastases. Since there is a significant learning curve for interpretive, operator-dependent procedures like ultrasonography and ablation, we believe that radiologist-performed IOUS represents theoretical best practice. As a result, interventional radiologists routinely perform IOUS and intra-operative ablations of liver metastases at our institution.
In this study, we sought to evaluate the utility of radiologist-performed IOUS by (1) conducting a retrospective chart review to determine how frequently the surgical plan is altered based on the radiologist’s intraoperative findings, and (2) prospectively implementing standardized processes of care in regards to optimizing preoperative imaging to determine if IOUS is still beneficial.
Materials and Methods
I Study Design and Population
This study consisted of two separate methodologies: a retrospective review of all patients who underwent hepatic resection for metastatic colorectal cancer (CRC) or neuroendocrine tumour (NET) from January 2012 to August 2013, and a prospective study of patients undergoing liver resection for the same from January 2014 to January 2016. All liver resections were performed by two fellowship trained hepatobiliary surgeons (SN and DJ) at Queen's University, Kingston, Ontario, Canada. All patients underwent resection of their primary malignancy prior to resection of their liver metastases. For the retrospective study, patient demographics, perioperative details, and clinicopathologic factors were collected from patient charts. For the prospective study, patients were consented to undergo both preoperative CT and MRI, one of which to be completed within 30 days prior to surgery, as well as IOUS. Similar demographic, operative and clinicopathologic variables were collected for the prospective cohort as the retrospective group. This study was approved by and conducted in accordance with our institutional ethics review board.
II Surgical Assessment and Operative Procedure
Patients were deemed to have resectable disease by the consultant surgeon based on the ability to resect all metastatic disease with negative margins while still maintaining an adequate predicted remnant liver to avoid liver failure. In selected patients, preoperative portal vein embolization was performed at the discretion of the hepatobiliary surgeon if there was a concern of inadequate residual liver volume. The complete examination of the liver was performed by palpation and ultrasonography to detect occult lesions and to delineate anatomic relationships between the tumors and vascular structures. For anatomic resections, hepatic arterial and portal venous inflow, as well as hepatic venous outflow, were often divided prior to parenchymal transection, which was almost exclusively performed with the Erbejet hydrodissector (Erbe, Atlanta, Georgia, USA) under low central venous pressure conditions. A Pringle maneuver was used selectively and only in the event of hemorrhage during hepatic transection. For selected patients, hepatic resection was combined with concurrent radio-frequency ablation (performed by the interventional radiologist) when all visible disease could not be treated with resection alone.
III Imaging Protocols
Multiphase CT and MRI liver protocol studies were reviewed. CT liver protocols at our institution were performed on GE LightSpeed VCT 64 (GE Healthcare, Chicago, Illinois, USA) and Aquilion One 320 (Canon Medical Systems, previously Toshiba, Otawara, Japan) CT scanners with the acquisition of an unenhanced liver followed by post-contrast imaging at 10s, 65s and 180s. MRI liver protocols were performed on an Avento 1.5T MRI scanner (Siemens Healthcare, Malvern Pennsylvania, USA) with the acquisition of the following sequences: coronal T2, axial T1 in and out of phase, axial T2, axial DWI (b0, b400 and b800), axial T2 with fat saturation, axial T1 with fat saturation pre and post gadolinium with Gadovist (Bayer, Leverkusen, Germany) at immediate arterial phase, 60s, 180s, and 300s. IOUS was performed by a single fellowship-trained interventional radiologist (AM) using a Philips CX50 ultrasound machine, and a combination of curvilinear 1-5 MHz and linear 4-12 MHz probes (Philips Healthcare, Andover, Massachusetts, USA).
IV Statistical Analysis
All statistical analysis was performed in Microsoft® Excel 2016 for Mac (Microsoft, Redmond, WA). A two-tailed T-test was used to test for significance for reduction in mean time between imaging and surgery in the prospective group versus the retrospective group. A chi-square test was used to test for significance in the change in operative plan as a result of IOUS between the prospective and retrospective cohorts.
Results
I Clinical, Radiographic and Surgical Characteristics of Patients
In the retrospective review, a total of 34 patients underwent 39 liver resections, compared to 24 patients undergoing 27 liver resections in the prospective study. Colorectal cancer was the dominant primary malignancy in both groups with comparable disease-free interval. While the mean age was similar between both groups, there were proportionally more females in the retrospective group compared to the prospective cohort (Table 1).
The burden of metastatic disease with respect to the size of the largest metastasis and number of lesions was similar between groups (Table 2), as was the extent of surgical resection (Table 3). As expected, the timing and frequency of MRI imaging were different between both groups. In the retrospective cohort, all patients underwent a preoperative CT scan, and 36% had a preoperative MRI with a mean time between most recent imaging and surgery of 47 days. In the prospective cohort, all patients underwent both preoperative CT and MRI with a significantly reduced mean time between most recent imaging and surgery of 20 days (p=0.001, Table 2).
Table 1: Characteristics of patients undergoing liver resection for metastatic disease before and after standardization of preoperative protocola.
|
|
Pre-standardization (n=34) |
Post-standardization (n=24) |
|
Patient-related Age (mean, range) <50 50-70 >70 |
63.6 (39-85) 3 (9%) 20 (59%) 11 (32%) |
62.4 (38-80) 3 (13%) 14 (58%) 7 (29%) |
|
Sex Female Male |
12 (39%) 22 (61%) |
4 (17%) 20 (83%) |
|
Disease-related Primary malignancy Colorectal cancer Neuroendocrine tumor |
31 (91%) 3 (9%) |
20 (83%) 4 (17%) |
|
Timing of Metastases Synchronous metastases Metachronous metastases Disease free interval (months) |
19 (56%) 15 (44%) 17 (3-46) |
12 (50%) 12 (50%) 21 (3-60) |
aValues are either reported as n (percentage) or mean (range).
Table 2: Radiographic characteristics of liver metastases and details of imaging for metastasectomies performed before and after standardization of preoperative protocola.
|
|
Pre-standardization (n=39) |
Post-standardization (n=27) |
|
Characteristics of Metastases Number of metastases Mean (range) <2 2-10 >10 |
3.3 (1-12) 16 (41%) 21 (54%) 2 (5%) |
4.2 (1-15) 6 (22%) 18 (67%) 3 (11%) |
|
Size of largest metastatic deposit (cm) Mean (range) <2 2-5 >5 |
2.56 (0-8) 15 (33%) 21 (53%) 3 (14%) |
3.5 (0.9-10.1) 5 (19%) 17 (63%) 5 (19%) |
|
Preoperative imaging Preoperative CT Preoperative MRI Preoperative CT and MRI Time from most recent imaging to surgery (days) |
39 (100%) 14 (36%) 14 (36%) 46.4 (7-126) |
27 (100%) 27 (100%) 27 (100%) 20.1 (1-98)*
|
aValues are either reported as n (percentage) or mean (range).
*p=0.001
Table 3: Surgical characteristics of operation for metastatic liver disease performed before and after standardization of preoperative protocol.
|
|
Pre-standardization |
Post-standardization |
|
Type of liver resectiona Right or left hepatectomy Extended right or left hepatectomy Minor anatomic resection Wedge resection Radiofrequency ablation (RFA) Aborted procedure Total no. of procedures + aborted procedures |
n (%) 10 (20%) 13 (26%) 6 (12%) 13 (26%) 5 (10%) 3 (6%) 50 (100%) |
n (%) 6 (18%) 8 (24%) 2 (3%) 9 (26%) 7 (21%) 2 (6%) 34 (100%) |
aMultiple procedures per patient included; denominator for proportion is total number of procedures + aborted procedures.
II Effect of IOUS on Intra-Operative Decision-Making
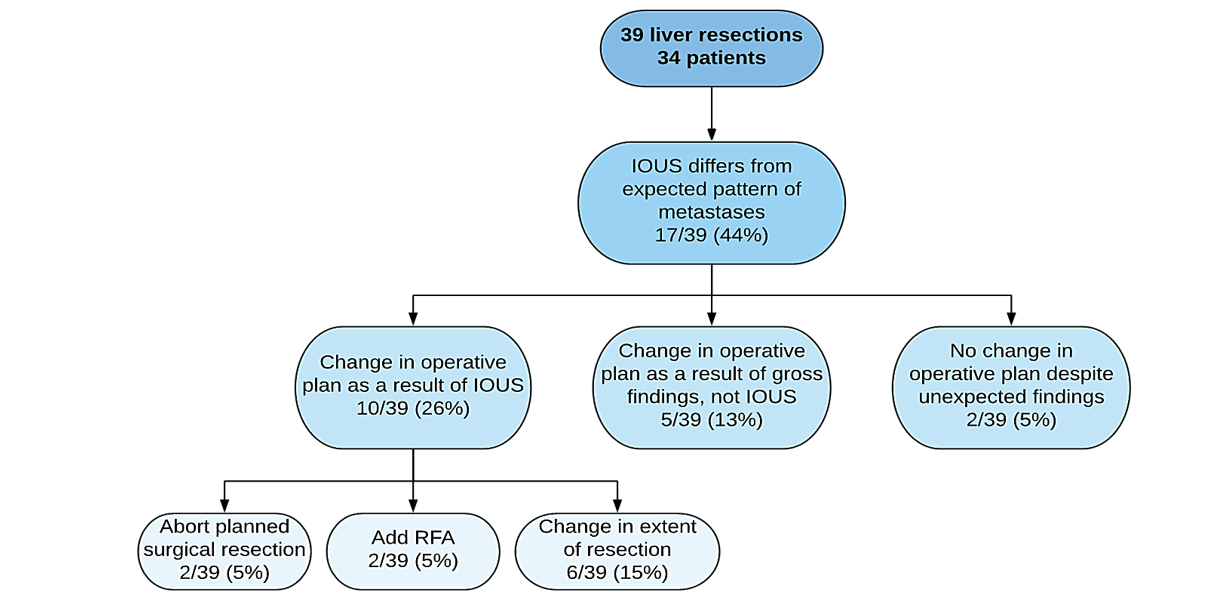
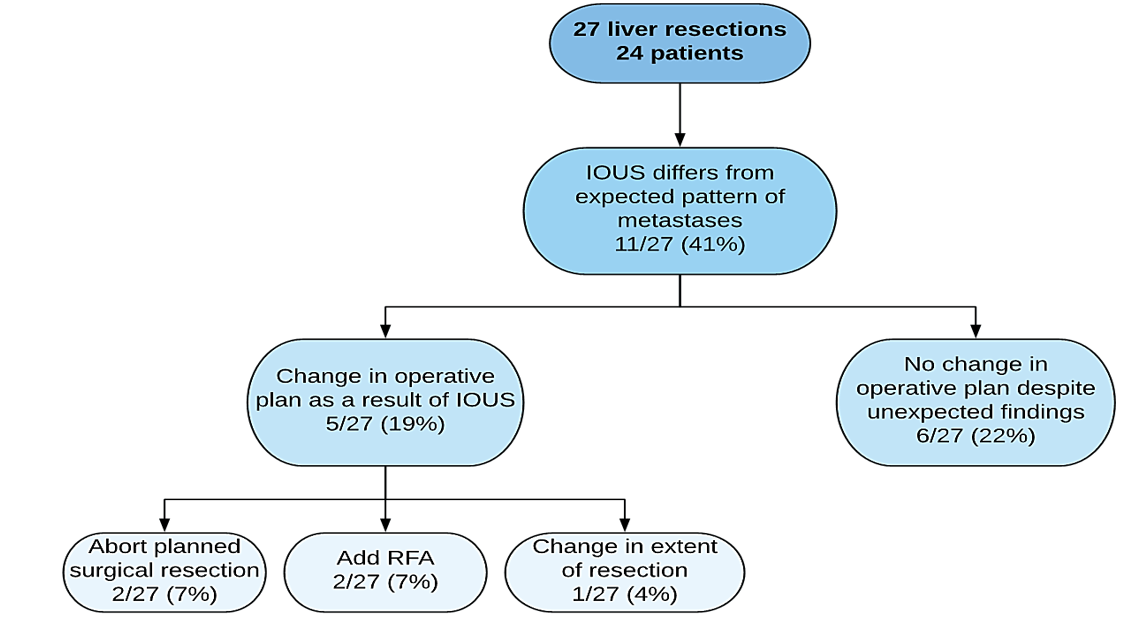
In the retrospective group, IOUS findings were different from the expected pattern of metastases described by preoperative imaging in 44% of cases (Figure 1). There was a change in operative plan in 39% of these patients; 13% was based on unexpected gross findings, and 26% was based specifically on IOUS findings. In the prospective group, results of IOUS differed from the expected pattern of metastases in 41% of cases (Figure 2). There was a change in the operative plan as a result of IOUS, specifically in 19% of cases (χ2=1.405, p=0.24).
Figure 1: Influence of IOUS on intraoperative decision-making (pre-standardization of preoperative protocol).
Figure 2: Influence of IOUS on intraoperative decision-making (post-standardization of preoperative protocol).
Discussion
Radiologist-performed IOUS offers the benefits of enhanced accuracy with specialist-interpreted ultrasound and real-time decision-making about interventional adjuncts. Harlow et al. suggest from their experience in treating breast carcinoma “that an individual facile with the technique and interpretation of ultrasound be present in the operating room” and that “surgeons who are in the early stages of their experience with ultrasound will be best served by having radiology assistance until they can reliably identify all lesions themselves in patients on whom this procedure is done” [10]. It is considered that after completion of general ultrasound training, the learning curve for screening liver metastases is a minimum of 25 intraoperative examinations, while the learning curve for interventional techniques such as radiofrequency ablation of liver lesions is at least 50 maneuvers [11].
Because one cannot assume that every surgeon has had sufficient training in IOUS to screen for liver metastases and make recommendations on adjunctive procedures, we believe that radiologist performed IOUS represents theoretical best practice. The collaborative management for patients undergoing liver metastasectomy may improve outcomes by optimizing the combination of resection and radiofrequency ablation of all lesions with curative intent in the operating room [12, 13].
Several articles have reported on the concordance between preoperative imaging and intraoperative findings (IOUS +/- visual and manual exploration), as well as the frequency with which the operative plan is amended based on these findings. One large retrospective study reports superior concordance between lesions seen on IOUS and those found on pathological examination as compared to those seen on CT or MRI [1]. Another review examined the effect of IOUS on changing the operative approach and found that the operative plan changed in up to 50% of cases based on IOUS findings [14]. The issue of radiologist specific IOUS was assessed in a recent prospective trial examining the impact of IOUS in hepatic resections for colorectal liver metastases. This study reported a change in operative plan in 42 of 117 (36%) procedures based on operative exploration (IOUS and gross intraoperative findings) with a change due to IOUS findings alone in 35/117 cases (30%) [15].
At our centre, based on non-standardized preoperative imaging and IOUS performed by a radiologist, we found that 26% of patients undergoing hepatic metastasectomy had a change in the operative plan based on IOUS findings. This result is similar to the results reported in other studies and indicates that IOUS can still be beneficial for liver resection for metastases.
Mandatory preoperative liver imaging using multidetector CT, MRI, or PET CT is listed as one of the quality indicators for patients undergoing hepatic resection for metastatic colorectal cancer that was derived using evidence-based review of the literature and an iterative consensus methodology of experts in the field [16]. Currently, MRI is the imaging technique that provides optimal detection of intrahepatic metastases [17, 18]. A recent meta-analysis on preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy reported that MRI, followed by CT, is the most appropriate imaging modality in the preoperative assessment of patients with colorectal liver metastases [19]. However, timely access to MRI can be a challenge in some centres, and MRI is still not included in some clinical guidelines for the management of colorectal liver metastases [20, 21]. In our retrospective review, while all patients underwent preoperative CT, the rate of preoperative MRI was only 36%.
Given the wide variability in the type and timing of preoperative imaging in our retrospective cohort, the utility of IOUS, as indicated by the rate of change in the operative plan, may be an overestimation of its actual usefulness. After prospectively establishing a standardized process of care for preoperative imaging where 100% of patients underwent both preoperative CT and MRI, with one imaging modality completed within a month of the surgery, the frequency of change in the operative plan based on IOUS decreased from 26% to 19%. These results suggest that standardized preoperative imaging protocols can mitigate the need to change the operative plan as a result of IOUS, and potentially improve surgical care, especially in the centres that do not perform IOUS.
The literature has shown that variation in processes of care may lead to increased rates of error. Improved standardization and communication between healthcare providers through the implementation of protocols and checklists can reduce patient harm [22]. Reducing variation in processes of care has been a cornerstone of improved performance and reliability over the past several decades in healthcare [23]. By virtue of standardization alone, it has been shown that the adoption of one appropriate specific management plan will yield superior results to those achieved by the random application of several individually equivalent approaches [24]. With respect to preoperative imaging for patients undergoing liver resection for metastases, clinical practice guidelines recommend mandatory preoperative imaging. However, the literature is unclear about whether both CT and MRI are needed [25] and what time interval between imaging and surgery is clinically acceptable.
There are some important limitations to our study worthy of mention. At the time of our prospective data collection, our institution was using MRI with extracellular gadolinium-based contrast agents (Gadovist) for the preoperative imaging of our patient sample. Currently, many institutions are using MRI with hepatobiliary gadolinium contrast agents (Primovist) or other liver-specific contrast media because these agents are more sensitive for detecting metastases [26, 27]. This new development in imaging may reduce the applicability of our findings as it relates to the utility of IOUS. Also, our patient sample in both retrospective and prospective cohorts is small and limited to two surgeons’ cases in a single institution. Despite these limitations, we believe that the findings of this study are still noteworthy as they highlight the potential utility of implementing standardized protocols for preoperative imaging to improve surgical care, as well as the fact that IOUS may still be an important adjunct in liver resection for metastatic disease.
In summary, we demonstrated that the radiologist-performed IOUS in the setting of hepatic metastasectomy provided important additional information that influenced the operative plan. Even after standardizing and improving the quality and timing of preoperative imaging, IOUS still led to a change in the operative plan in nearly one in every five patients. Our results comparing the effect of IOUS in changing the operative plan before and after improving processes of care serve as a basis for quality improvement strategy at our institution and may prompt other institutions to consider implementing a standardized preoperative imaging protocol.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 20, Apr 2020Accepted: Mon 04, May 2020
Published: Tue 12, May 2020
Copyright
© 2023 Sulaiman Nanji. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.03.02
Author Info
Alexandre Menard Diederick Jalink Lauren O’Malley Shannon Wong Sulaiman Nanji
Corresponding Author
Sulaiman NanjiDepartment of Surgery, Kingston Health Sciences Centre, Kingston, Ontario, Canada
Figures & Tables
Table 1: Characteristics of patients undergoing liver resection for metastatic disease before and after standardization of preoperative protocola.
|
|
Pre-standardization (n=34) |
Post-standardization (n=24) |
|
Patient-related Age (mean, range) <50 50-70 >70 |
63.6 (39-85) 3 (9%) 20 (59%) 11 (32%) |
62.4 (38-80) 3 (13%) 14 (58%) 7 (29%) |
|
Sex Female Male |
12 (39%) 22 (61%) |
4 (17%) 20 (83%) |
|
Disease-related Primary malignancy Colorectal cancer Neuroendocrine tumor |
31 (91%) 3 (9%) |
20 (83%) 4 (17%) |
|
Timing of Metastases Synchronous metastases Metachronous metastases Disease free interval (months) |
19 (56%) 15 (44%) 17 (3-46) |
12 (50%) 12 (50%) 21 (3-60) |
aValues are either reported as n (percentage) or mean (range).
Table 2: Radiographic characteristics of liver metastases and details of imaging for metastasectomies performed before and after standardization of preoperative protocola.
|
|
Pre-standardization (n=39) |
Post-standardization (n=27) |
|
Characteristics of Metastases Number of metastases Mean (range) <2 2-10 >10 |
3.3 (1-12) 16 (41%) 21 (54%) 2 (5%) |
4.2 (1-15) 6 (22%) 18 (67%) 3 (11%) |
|
Size of largest metastatic deposit (cm) Mean (range) <2 2-5 >5 |
2.56 (0-8) 15 (33%) 21 (53%) 3 (14%) |
3.5 (0.9-10.1) 5 (19%) 17 (63%) 5 (19%) |
|
Preoperative imaging Preoperative CT Preoperative MRI Preoperative CT and MRI Time from most recent imaging to surgery (days) |
39 (100%) 14 (36%) 14 (36%) 46.4 (7-126) |
27 (100%) 27 (100%) 27 (100%) 20.1 (1-98)*
|
aValues are either reported as n (percentage) or mean (range).
*p=0.001
Table 3: Surgical characteristics of operation for metastatic liver disease performed before and after standardization of preoperative protocol.
|
|
Pre-standardization |
Post-standardization |
|
Type of liver resectiona Right or left hepatectomy Extended right or left hepatectomy Minor anatomic resection Wedge resection Radiofrequency ablation (RFA) Aborted procedure Total no. of procedures + aborted procedures |
n (%) 10 (20%) 13 (26%) 6 (12%) 13 (26%) 5 (10%) 3 (6%) 50 (100%) |
n (%) 6 (18%) 8 (24%) 2 (3%) 9 (26%) 7 (21%) 2 (6%) 34 (100%) |
aMultiple procedures per patient included; denominator for proportion is total number of procedures + aborted procedures.
References
- D'Hondt M, Vandenbroucke Menu F, Preville Ratelle S, Turcotte S, Chagnon M et al. (2011) Is intra-operative ultrasound still useful for the detection of a hepatic tumour in the era of modern pre-operative imaging? HPB (Oxford) 13: 665-669. [Crossref]
- Ferrero A, Langella S, Giuliante F, Viganò L, Vellone M et al. (2013) Intraoperative liver ultrasound still affects surgical strategy for patients with colorectal metastases in the modern era. World J Surg 37: 2655-2663. [Crossref]
- Conlon R, Jacobs M, Dasgupta D, Lodge JP (2003) The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound 16: 211-216. [Crossref]
- Machi J, Isomoto H, Kurohiji T, Yamashita Y, Shirouzu K et al. (1991) Accuracy of intraoperative ultrasonography in diagnosing liver metastasis from colorectal cancer: evaluation with postoperative follow-up results. World J Surg 15: 551-556. [Crossref]
- Wagnetz U, Atri M, Massey C, Wei AC, Metser U (2011) Intraoperative ultrasound of the liver in primary and secondary hepatic malignancies: comparison with preoperative 1.5-T MRI and 64-MDCT. AJR Am J Roentgenol 196: 562-568. [Crossref]
- D'Onofrio M, Gallotti A, Martone E, Nicoli L, Mautone S et al. (2009) Is intraoperative ultrasound (IOUS) still useful for the detection of liver metastases? J Ultrasound 12: 144-147. [Crossref]
- Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW et al. (2014) Small (</=1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 271: 748-760. [Crossref]
- Scharitzer M, Ba Ssalamah A, Ringl H, Kolblinger C, Grunberger T et al. (2013) Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol 23: 2187-2196. [Crossref]
- Kartalis N, Brismar TB, Mihocsa L, Isaksson B, Albiin N (2011) The added value of contrast-enhanced ultrasound in patients with colorectal cancer undergoing preoperative evaluation with extensive gadobenate dimeglumine liver MRI. Eur Radiol 21: 2067-2073. [Crossref]
- Harlow SP, Krag DN, Ames SE, Weaver DL (1999) Intraoperative ultrasound localization to guide surgical excision of nonpalpable breast carcinoma. J Am Coll Surg 189: 241-246. [Crossref]
- Parks KR, Hagopian EJ (2014) Introduction: the importance of ultrasound in a surgical practice. In: Hagopian EJ, Machi J, eds. Abdominal Ultrasound for Surgeons. New York: Springer: 3-6.
- Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D (2009) A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer 45: 1748-1756. [Crossref]
- Eltawil KM, Boame N, Mimeault R, Shabana W, Balaa FK et al. (2014) Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol 110: 734-738. [Crossref]
- Kruskal JB, Kane RA (2006) Intraoperative US of the liver: techniques and clinical applications. Radiographics 26: 1067-1084. [Crossref]
- Sietses C, Meijerink MR, Meijer S, van den Tol MP (2010) The impact of intraoperative ultrasonography on the surgical treatment of patients with colorectal liver metastases. Surg Endosc 24: 1917-1922. [Crossref]
- Dixon E, Armstrong C, Maddern G, Sutherland F, Hemming A et al. (2009) Development of quality indicators of care for patients undergoing hepatic resection for metastatic colorectal cancer using a Delphi process. J Surg Res 156: 32-38.e1. [Crossref]
- Legou F, Chiaradia M, Baranes L, et. al. Imaging strategies before beginning treatment of colorectal liver metastases. Diagn Interv Imaging 95: 505-512. [Crossref]
- Maegerlein C, Fingerle AA, Souvatzoglou M, Rummeny EJ, Holzapfel K (2015) Detection of liver metastases in patients with adenocarcinomas of the gastrointestinal tract: comparison of 18F-FDG PET/CT and MR imaging. Abdom Imaging 40: 1213-1222. [Crossref]
- Van Kessel CS, Buckens CFM, van den Bosch MA, van Leeuwen MS, van Hillegersberg R et al. (2012) Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol 19: 2805-2813. [Crossref]
- Barua B (2017) Waiting your turn: wait times for health care in Canada, 2017 report.
- National Institute for Health and Care Excellence (2011) Colorectal cancer: diagnosis and management. Clinical guideline [CG131].
- Gawande A (2009) The checklist manifesto: how to get things right. New York: Metropolitan Books.
- Committee on Patient Safety and Quality Improvement (2015) Committee Opinion No. 629: Clinical guidelines and standardization of practice to improve outcomes. Obstet Gynecol 125: 1027-1029. [Crossref]
- Clark SL, Nageotte MP, Garite TJ, Freeman RK, Miller DA et al. (2013) Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol 209: 89-97. [Crossref]
- Mainenti PP, Romano F, Pizzuti L, Segreto S, Storto G et al. (2015) Non-invasive diagnostic imaging of colorectal liver metastases. World J Radiol 7: 157-169. [Crossref]
- Neri E, Bali MA, Ba Ssalamah A, Boraschi P, Brancatelli G et. al. (2016) ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol 26: 921-931. [Crossref]
- Jhaveri K, Cleary S, Audet P, Balaa F, Bhayana D et al. (2015) Consensus statements from a multidisciplinary expert panel on the utilization and application of a liver-specific MRI contrast agent (gadoxetic acid). AJR Am J Roentgenol 204: 498-509. [Crossref]
