Surgical Correction of Genitourinary Disorders Using the Self-Assembly Tissue Engineering
Surgical Correction of Genitourinary Disorders Using the Self-Assembly Tissue Engineering
A B S T R A C T
Urologic and gynaecologic patients often manifest congenital and/or acquired tissue and organ dysfunctions that require surgical reconstruction to recreate the normal genitourinary system functions. Such reconstruction is still a challenge due to the limited availability of suitable tissues, especially for severe urethral and vaginal replacement. Traditional intervention methods have varying degrees of donor site morbidity or inherent side effects.
Interestingly, tissue engineering is a growing field that aims to replace or regenerate these dysfunctional tissues and organs with autologous cells, biomaterials, or a combination of both. Experience gained from tissue engineering suggests that the use of acellular matrices alone is not successful in supporting tissue growth over large surfaces. Cellular constituents need to be isolated, cultured and grown in vitro on scaffolds, then transplanted in vivo, in order to achieve successful three-dimensional tissue regeneration.
Biomaterials are the backbone for cell-seeded reconstruction of the genitourinary tissues. Several research teams have explored a different cell culture approach based on self-assembly.
This innovative technique relies on the capacity of cells cultured in the presence of ascorbate to secrete and deposit their own extracellular matrix forming a tissue-like substance. The mechanical and physical strength properties of these reconstructed tissues are similar to that of natural native tissues in certain models. Tissue engineered substitutes for urethral and vaginal mucosa were produced and subcutaneously grafted with success opening the way to new therapeutic strategies to correct genitourinary defects.
Keywords
Tissue engineering, urethra, vagina, urology, gynaecology, epithelium
What are the genitourinary pathologies in need for a tissue engineering solution?
Virtually the whole genitourinary tract can be affected by several pathologies, which could find a solution using tissue engineering. Nevertheless, in this review we want to focus only on diseases and reconstructive strategies affecting the urethra and vagina.
Disorders affecting the urethra
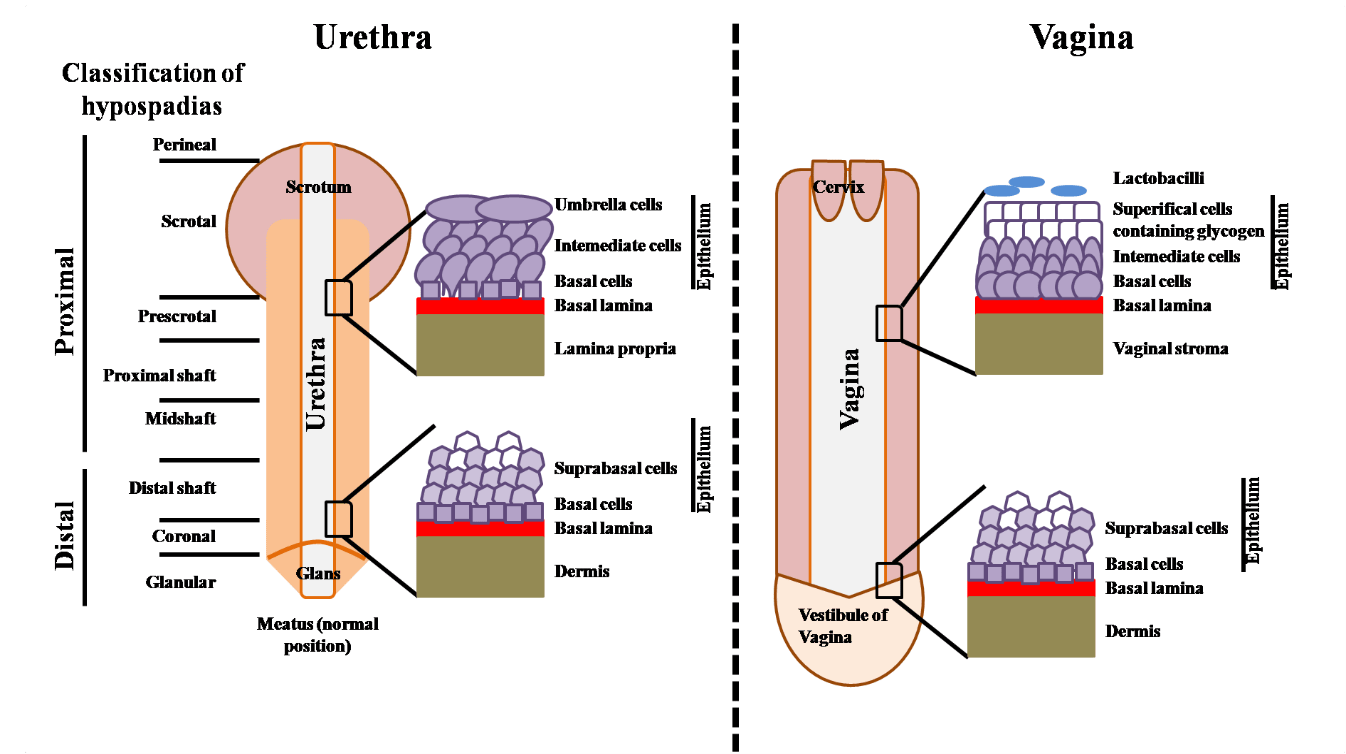
Urologic patients often show up with congenital and/or acquired tissue and organ dysfunctions requiring surgical reconstruction to re-establish a normal genitourinary system function. Hypospadias (Figure 1) and urethral stricture disease are amongst the most widespread. Hypospadias is the most frequent penile malformation (73% of all congenital penile anomaly): one out of 250 newborn males are affected and several studies have reported an increasing prevalence of hypospadias in humans [1-7]. Hypospadias prevalence have been showed to be low in Asia and high in North America [8]. Studies showed that hypospadias heritability is estimated to 57-77% and are equally transmitted through the maternal and paternal sides of the family [9]. The recurrent risks for brothers and sons in the same family being similar, genetic and shared environment factors should play a main role in familial hypospadias [10]. Furthermore, maternal hypertension, oligohydramnios, and premature delivery are linked with sever hypospadias suggesting a main role of underlying placental insufficiency potentially through inadequate levels of human chorionic gonadotropin (hCG) to the fetus [11]. In this clinical condition, the urethral opening is inadequately positioned below the tip of the glans penis and could be positioned anywhere along the ventral side of the penis (Figure 1). Depending on where the urethral opening is located, the severity of the hypospadias can be minor (close to the glans) or severe (meatus is close to the scrotum or within it) and often requires surgery [12-14]. Frequent and impactful, hypospadias is an important health issue and can be a substantial burden on health-care resources [15]. Indeed, the most severe cases could require subsequent surgeries due to complications such as complete dehiscence, stenosis or fistulae [16].
Figure 1: Schema of urethra and vagina anatomic and histologic features. Left: Urethra, classification of hypospadias on the basis of the position of the meatus is indicated. Right: Vagina.
Male urethral stricture disease most commonly results from injury, instrumentation, infection, non-infectious inflammatory conditions of the urethra, and after prior hypospadias surgery. Less common causes include congenital urethral strictures and those resulting from malignancy. It occurs at a rate as high as 0.6% and results in more than 6,000 inpatient visits yearly in the U.S. Yearly office visits for urethral stricture numbered almost 1.8 million between 2005 and 2013. The total cost of urethral stricture diseases in USA in 2010 was almost $300 millions. A diagnosis of urethral stricture increased health care expenditures by more than $6,000 per individual yearly [17]. Patients with urethral stricture disease appear to have a high rate of urinary tract infection (41%) and incontinence (11%) [18]. A wide variety of tissues, such as skin grafts, bladder and oral mucosa, have been used for urethral repair [19]. However, all of these substitutes have limitations compared to autologous urethral tissue, which can lead to complications (e.g. stricture formation, graft failure) [20-22]. Furthermore, the amount of tissue that can be harvested from a donor site is limited, which can be problematic, especially in the case of long defects. For oral mucosa, the actual gold standard in clinic for challenging cases, this would mean a two-stage surgery and mucosa from the inside of both cheeks being harvested, causing significant discomfort postoperatively. Since mucosa cannot be harvested twice from the same site, this limits surgical options if a complication occurs. To overcome these difficulties, alternative methods for urethral reconstruction have been explored.
Current tissue engineering strategies for urologic tissue reconstruction
Tissue engineering (TE) is an emerging field offering the possibility of providing true biological substitutes with patient-specific properties to restore the structure and function of pathologically altered tissues. Several groups have attempted TE urethral substitution by using acellular matrices such as Bladder Acellular Matrix Graft (BAMG) and Small Intestinal Submucosa (SIS), or cellularized matrices [23-32]. These matrices are prepared from native tissues by decellularizing and sterilising it. A major issue concerning acellular matrices, as shown in rabbits by Dorin et al., is that urothelial regeneration in acellular graft is limited to 0.5 cm, which compromises success in more complex cases, such as long strictures [33]. Synthetic polymers have also showed advantages (PLLA and PLGA) to form tridimensional (3D) organs biocompatible at a low cost with a control of mechanical properties. However, a synthetic scaffold doesn’t allow a correct epithelial cell differentiation into a well-organized tissue. Long-term experiment hasn’t been achieved because of urothelial low differentiation and apparition of microfistulas. TE matrices containing autologous cells in addition to extracellular matrix are more promising. The main advantage of this method is that a large autologous cell graft having the ability to grow in vivo without rejection can be created with limited material, such as a piece of oral mucosa. Moreover, studies have reported that stem cells can be obtained from urine, making this approach even more polyvalent [34, 35]. Despite significant progress in the urethral TE field, very few teams have proceeded to clinical trials and published their results to date [36]. However, the four clinical trials so far conducted present good results in a limited number of patients with long-segment and/or complex stricture disease [37-41]. Although these models are certainly far from an “off-the-shelf” alternative, with consistently reproducible outcomes, this could offer an alternative for challenging cases requiring long-segment urethral replacement [42]. However, after long periods in culture to obtain well-differentiated tissues, exogenous matrices become difficult to manipulate and lose mechanical and physical properties.
Disorders affecting the vagina
Patients presenting congenital or acquired malformations of the reproductive tract are often in need of extensive surgical reconstruction. Cloacal and bladder exstrophy, in which the anatomical structures of the pelvis (including bladder, genitalia, and colon) fail to fuse in the midline, are examples of such malformations. Children afflicted with intersex disorders, such as congenital adrenal hyperplasia and cloacal anomalies, can have significant anatomical defects and are often in need of extrinsic tissue sources for reconstructive surgery. Müllerian agenesis, also known as Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome is the most common anomaly of the Müllerian duct development, which results in vaginal agenesis. MRKHS covers a range of anomalies that are all associated with vaginal and uterine abnormalities (1500 to 4000 female births), but might also have other associated findings [43, 44]. Pelvic floor disorders affect nearly one-third of premenopausal women and half of postmenopausal women [45, 46]. Likewise, in the US, numbers of surgical interventions for pelvic organ prolapse and urinary incontinence are not negligible. In 2010, approximately 300 000 women underwent surgical procedures to repair pelvic organ prolapse (POP); 33% of these were performed with mesh, with 25% of mesh placed transvaginally. Approximately 260 000 women underwent incontinence surgery, with 80% of repairs performed using a mesh device, with a direct cost of over $1 billion dollars annually in the United States [45, 47-50]. The likelihood of a woman requiring surgery for pelvic organ prolapse or urinary incontinence in her lifetime is 10–20% [51-53]. Evidence of efficacy for these mesh products is lacking, and rates of complications such as erosions, pain, infections, and vaginal shrinkage are unacceptably high at around 10 % [54-59]. Those complications will often require interposition tissue like a graft, to cover the defect. The U.S. Food and Drug Administration (FDA) ordered the manufacturers of all remaining surgical mesh products indicated for the transvaginal repair of pelvic organ prolapse to stop selling and distributing their products in the U.S. immediately [60]. Women with cervical, uterine, ovarian, rectal, vaginal, or bladder cancer may require partial or total vaginal resection, and they may need partial or total vaginal replacement for the recovery of sexual function and restoration of native anatomy. Vaginal stenosis could also happen after radiotherapy treatment to cure colorectal and cervical cancers (in 80% of treated women) [61]. It is characterized by a decrease in the length and diameter of the vagina, followed by scar tissue formation; for example, 4,000 women in Canada could be affected post-radiotherapy. Moreover, vaginal stenosis or strictures could also happen in the case of: vaginal atrophy, hypoestrogenic states, inflammatory and autoimmune diseases and chemical vaginitis [61]. Transient and long-term injuries to the vagina and its supportive tissues have also been documented following vaginal delivery, and most parous women have some anatomical evidence of disrupted support [62-64]. Gender reassignment is also a challenge and a long-term perspective of vaginal tissue engineering. Thus, the search for an ideal tissue remodelling material for uro-gynaecological repair is ongoing.
Current alternative strategies, tissue engineering for vaginal regenerative medicine
Vaginal anomalies represent a major women’s health issue because nearly 1% of women will suffer from these pathologies resulting in significant psychological impacts. Interestingly, tissue engineering is a field that aims to replace or regenerate these dysfunctional tissues and organs with autologous cells, biomaterials, or a combination of both. Successful vaginal reconstruction in these patients depends largely on the use of a sufficiently abundant tissue substrate that adequately performs the physiological functions of the vagina. Past techniques have often relied on autologous tissues such as intestine or skin, which are often associated with complications due to the inherent physiological differences of these substrates. In an attempt to improve these results, a variety of biodegradable substitutes, including collagen matrices and decellularized bladder submucosa, have been used for vaginal replacement [65]. Reconstructions using theses substitutes have usually been unsuccessful owing to functional, structural, mechanical, or biocompatibility problems. The use of the patient’s vaginal tissue for reconstruction would provide the most elegant and successful solution, but this has frequently not been feasible because of the relative paucity of healthy vaginal tissue for autologous grafting. A tremendous clinical need exists for the development of technologies to facilitate the regeneration of injured or diseased tissues and organs. The unrelenting prevalence of trauma, congenital defects and diseases, such as cancer, drives the demand, which becomes increasingly urgent as the global population expands and ages. A wide variety of tissues and organs would benefit from engineering-based repair or regeneration. Several graft materials have been used to line the surgically created neovaginal cavity, including myocutaneous flaps or intestinal segments, full-thickness or split-thickness skin grafts, amniotic membrane, peritoneum, decellularized matrices, oral mucosa and vaginal epithelial tissues [32, 66-73]. These techniques are associated with graft contracture and/or stenosis that may require long-term dilatation. Oral mucosa vaginoplasties are associated with donor site morbidities due to the large tissue volume being harvested for creating the neovagina. Furthermore, the amount of tissue that can be harvested from a donor site is limited, which can be problematic, especially for large defects. To overcome these difficulties, alternative methods for vaginal reconstruction have been explored. Few groups have attempted TE vaginal reconstruction using acellular and cellularized matrices from natural or synthetic origin [74-79]. The tissues were transplanted into mice, rabbits or women. But more preclinical and clinical studies are required due to the limited number of subjects enrolled in those studies and it remains difficult to determine if the optimal technique has been used.
Self-assembly tissue engineering
A new type of extracellular matrix (ECM) has been explored: the one produced by the “self-assembly” method. Major discoveries and therapeutic achievements have been made possible because of this unique technique which allows production of reconstructed tissues free of any foreign matrices [80]. Indeed, the use of exogenous biomaterials may lead to immunologic and foreign body reactions and transmission of infections. This technique relies on the capacity of the cells cultured in the presence of ascorbic acid to secrete and deposit their own ECM to form cohesive sheets of cells and collagen (Figure 2). Whereas most biomaterials lose their mechanical and physical strength properties in culture, the properties of self-assembled tissues are roughly similar or even exceed that of natural native tissues in certain models due to a stabilization of metalloproteinases [81, 82]. Using the self-assembly technique, it has been possible to reconstruct different cellularized models from various stromal cells originating from skin, fat, bladder and vagina which present an excellent mechanical strength [83-86].
Figure 2: Schema of production of reconstructed tissues using the self-assembly technique: A- Reconstructed flat model; B- Reconstructed tubular model. Same techniques were used to produce urethra and vagina but with organ-specific mesenchymal and epithelial cells. Some elements of the protocol were adapted (e.g.: culture medium used to culture for vaginal cells is different from the one used to culture urologic cells).
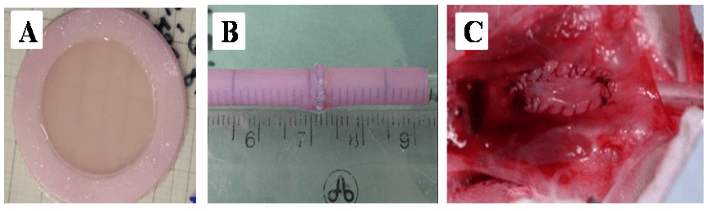
Figure 3: Macroscopic view of reconstructed tissues: A- Reconstructed flat model. B-Reconstructed tubular model at the end of the maturation period in the bioreactor. Tubes are easy to handle and resistant to sutures, using resorbable 7-0 Maxon. C- Urethral model implanted on rabbit urethra as a patch (half diameter).
Self-assembly to reconstruct genitourinary tissues
A TE genitourinary tubular graft was successfully assembled (Figure 3). Engineered tissues were subjected to maturation in dynamic conditions in a bioreactor and were characterized for histological and mechanical properties [81, 87]. This construct has outstanding histological organisation and very good mechanical resistance; hence it became obvious to use this tubular urological tissue as a urethral substitution model. The advantage of this cell-based technique to produce tissue-engineered urethra by self-assembly technique is that it contains mesenchymal cells that communicate with epithelial cells either through release of cytokines and growth factors or cell-cell contact. Although the best cell source for bioengineering urethras by self-assembly is mesenchymal cells from the patients target organ, it is unpractical as there are risks associated with the biopsy of the urethra such as creating a fistula.
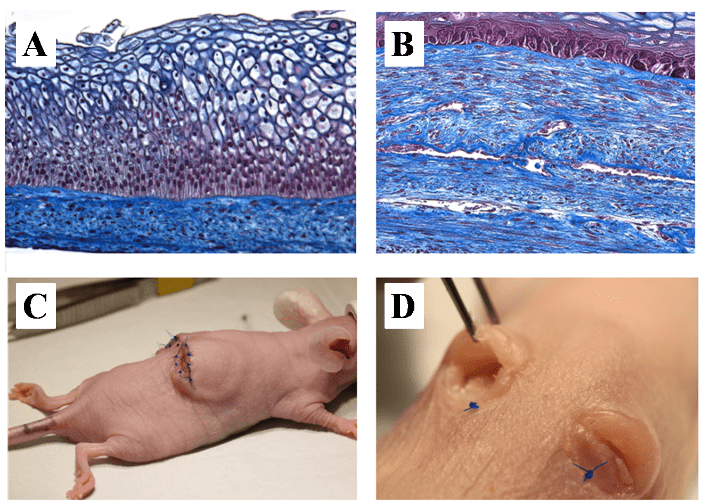
Hence another source of easily obtained cells should be considered. We have initially developed the urethral construct from dermal fibroblasts (DF) but bladder mesenchymal cells have also been used with success [81, 88]. Adipose-derived stem/stromal cells (ASC)-derived biomaterial for bladder regeneration were also successfully engineered [89]. ASC are easily harvested from a small sample of subcutaneous fat and yield a high proportion of multipotent cells (about 2%) [90-92]. They have immunomodulatory and angiogenic properties that could potentially improve the quality of the construct [93, 94]. Additionally, endothelial cells (EC) could be added to the model and form an advanced 3D capillary-like network to improve transplantation outcomes, avoiding ischemic events after the graft [95, 96]. This is the main obstacle observed in the development of a thick biomaterial or reconstructed tissue in vitro that can be used in human clinical applications. Indeed, neovascularization of a tissue is a slow process that can take over 15 days for a 1mm-thick tissue [97, 98]. The vascular network already present in the conventional autologous graft can reconnect to the host’s bloodstream in 4 days (inosculation) [99-103]. The solution to the problem of re-vascularization of in vitro reconstructed tissues could reside in the reconstruction of a capillary-like network within the graft prior to implantation. Encouraging results were obtained with inosculation on day 4 of transplantation with non-transfected EC (Figure 4) [95, 104]. Besides improving graft-take, the early capillary network could also help to clear the blood cells, fibrin and growth factors like TGF-β1, which can lead to fibrosis and disease recurrence [105]. Using a similar technique, a vaginal mucosa (VM) substitute was recently produced. The best cell source for bioengineering VM by self-assembly is cells from the patient’s target organ, therefore cells from human vagina were extracted and used for the reconstruction. The reconstructed tissues presented a good mechanical resistance and elasticity. They displayed a well-differentiated epithelium with expression of oestrogen receptor-beta and glycogen storage (Figure 5A) and could be preendothelialized (Figure 5B). Furthermore, it has been grafted subcutaneously in mice. The tissue survived with no sign of necrosis during the 2 months of in vitro reconstruction and 3 weeks after implantation [86].
Figure 4: Effects of endothelial cells on reperfusion of the graft after subcutaneous implantation in mice: Macroscopic aspect. Tubular urethral models reconstructed without (on the left side) or with endothelial cells (EC, right side). Macroscopically, UM with EC was clearly more vascularized at 14 days in the UM. At 28 days, tubes were well incorporated in mice tissues in both models but looked healthier with EC (graft take).
Figure 5: Vaginal model. A- Photograph of a a slice of reconstructed vaginal model stain by the Masson’s trichrome protocol. Epithelial layers are clearly visible, especially the one which contain glycogen required to maintain adequate pH for lactobacilli proliferation. B- Photograph of a slice of reconstructed vaginal model with endothelial cells (EC) stain by the Masson’s trichrome protocol. Capillary-like network is visible throughout the engineered tissue. C- Macroscopic view of the grafted vaginal model on the back of a mouse. D- Macroscopic appearance of the grafted material 3 weeks post-implantation.
Translation to rabbit as an animal model
The absence of exogenous materials and the autologous property of this self-assembled model represent significant advantages comparatively to other available grafts. Therefore, an autologous TE urethra was implanted in a rabbit model, the gold standard animal model to study penile surgery [95, 106, 107]. Technical adjustments were necessary to replicate results when using rabbit cells [108]. Hence, experimental settings should be altered to include organ-specific mesenchymal cells extracted from the bladder. This led to the formation of more elastic tissues and more differentiated urothelium devoid of K14 expression [88].
Urethral reconstruction by the self-assembly approach: limitations and perspectives
Urethral anomalies represent a major public health issue because nearly 1% of men are suffering from these pathologies that can have significant psychological impact. Treatment involves surgical correction and current therapeutic options are associated with morbidities and lack of durable long-term results. The solution may lie in the reconstruction of an autologous urethra from a small biopsy on the patient, in vitro reconstruction and its later implantation. A purely autologous tissue, with less fibrosis, would have a better function. The presence of cells at implantation would provide better growth potential, especially for pediatric patients. The urethral substitute produced by the self-assembly protocol is fully autologous and free of exogenous materiel, it can be pre-endothelialized and therefore possess, before implantation, histological and mechanical near-native features. Thus, a reduction in adverse events can be expected following the graft of this living engineered urethral tissue, which should grow as the child ages. It would attenuate the associated morbidity for the patients, at the implantation and harvesting sites, and decrease the financial burden of urethral anomalies on the healthcare system. Even if this model is associated with many advantageous characteristics, preparation time of the graft is not negligible. From cell culture to complete maturation, the reconstruction process takes 3 months. However, penile anomalies are chronic pathologies and surgical correction is made on an elective basis. Most patients must wait months before being operated. Consequently, additional delay to surgical correction does not represent a major inconvenience.
The necessity of a skin, bladder or adipose tissue biopsy is also a point to keep in mind, even if this intervention is simple and minimally invasive for skin and fat. Nevertheless, in the future, induced pluripotent stem cells (iPS), which can be produced from the blood of the patient, could be differentiated into the entire cell types required to reconstruct urethral substitutes. This new technology could avoid the invasive procedures such as biopsy harvesting. Nevertheless, it is absolutely required to control the differentiation step for all cell types used to avoid misdifferentiation and potential development of tumours [109].
The substantial expense related to the fabrication of this biological graft material should be taken in consideration, but it would be fully autologous, an advantageous characteristic for the patients. The current gold standard method is already associated with substantial expenses such as the frequent need for a surgery to be performed in 2 stages (2 different anesthesia, many months apart), the additional morbidity to the patient and the absence from work for a second major penile surgery (6 weeks each time).
The self-assembly approach to reconstruct urological tissues without biomaterial would open the door to the reconstruction of ureters, corpus spongiosum and cavernosa of the penis, which would significantly improve the quality of life for patients severely handicapped by congenital malformations of the genitals.
Vaginal reconstruction by the self-assembly approach: limitations and perspectives
The same limitations and perspective described for the urethral model apply to the vagina model. The development of an autologous vaginal mucosa reconstructed by TE would be a major advance in the field of uro-gynaecology and would also have a considerable clinical impact. To provide non-immunogenic replacement tissues, autologous and biomaterial-free, in order to circumvent the shortage of available tissues, is the main sought benefit of the reconstruction of vaginal tissue using patients’ cells with the self-assembly approach. Surgical reconstruction using the self-assembly method could significantly improve patient quality of life and may potentially decrease the financial burden of vaginoplasty on the healthcare system.
Conclusion
Genitourinary tissues were developed using the self-assembly approach using human organ-specific cells and they were implanted into animals with success. They constitute a promising avenue for surgical correction of various defects from congenital or acquired origins.
Conflicts of interest
The authors declare no potential conflicts of interest.
Acknowledgement
S. Bolduc is the recipient of a Canadian Urological Association Scholarship, a Canadian Institutes of Health Research Grant (#258229) and a Fonds Merck – Fondation de l’Université Laval Research Grant.
Article Info
Article Type
Review ArticlePublication history
Received: Mon 05, Aug 2019Accepted: Fri 06, Sep 2019
Published: Mon 16, Sep 2019
Copyright
© 2023 Stéphane Bolduc. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2019.03.01
Author Info
Ève Pellerin Christophe Christophe Emil Grammond Stéphane Chabaud Stéphane Bolduc Weronika Jakubowska
Corresponding Author
Stéphane BolducCentre de recherche en organogénèse expérimentale, LOEX, Regenerative Medicine Division, CHU de Québec Research Center, Quebec, QC, Canada
Figures & Tables


References
- Nelson CP, Park JM, Wan J, Bloom DA, Dunn RL et al. (2005) The increasing incidence of congenital penile anomalies in the United States. J Urol 174: 1573-1576. [Crossref]
- Blaschko SD, Cunha GR, Baskin LS (2012) Molecular mechanisms of external genitalia development. Differentiation 84: 261-268. [Crossref]
- Nordenvall AS, Frisen L, Nordenstrom A, Lichtenstein P, Nordenskjold A (2014) Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol 191: 783-789. [Crossref]
- Czeizel A (1985) Increasing trends in congenital malformations of male external genitalia. Lancet 1: 462-463. [Crossref]
- Paulozzi LJ, Erickson JD, Jackson RJ (1997) Hypospadias trends in two US surveillance systems. Pediatrics 100: 831-834. [Crossref]
- Baskin LS, Himes K, Colborn T (2001) Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 109: 1175-1183. [Crossref]
- Wilcox D, Snodgrass W (2006) Long-term outcome following hypospadias repair. World J Urol 24: 240-243. [Crossref]
- Springer A, van den Heijkant M, Baumann S (2016) Worldwide prevalence of hypospadias. J Pediatr Urol 12: 152 e151-e157. [Crossref]
- van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV et al. (2012) Aetiology of hypospadias: a systematic review of genes and environment. Human Reprod Update 18: 260-283. [Crossref]
- Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Christensen K et al. (2008) Familial aggregation of hypospadias: a cohort study. Am J Epidemiol 167: 251-256. [Crossref]
- Huisma F, Thomas M, Armstrong L (2013) Severe hypospadias and its association with maternal-placental factors. Am J Med Genet A 161A: 2183-2187. [Crossref]
- Snodgrass W, Macedo A, Hoebeke P, Mouriquand PD (2011) Hypospadias dilemmas: a round table. J Pediatr Urol 7: 145-157. [Crossref]
- Mouriquand PD, Persad R, Sharma S (1995) Hypospadias repair: current principles and procedures. Br J Urol 3: 9-22. [Crossref]
- Manzoni G, Bracka A, Palminteri E, Marrocco G (2004) Hypospadias surgery: when, what and by whom? BJU Int 94: 1188-1195. [Crossref]
- Ortqvist L, Fossum M, Andersson M, Nordenström A, Frisén L et al. (2015) Long-term followup of men born with hypospadias: urological and cosmetic results. J Urol 193: 975-981. [Crossref]
- Rompre MP, Nadeau G, Moore K, Ajjaouj Y, Braga LH et al. (2013) Learning curve for TIP urethroplasty: A single-surgeon experience. Can Urol Assoc J 7: E789-E794. [Crossref]
- Davis NF, Quinlan MR, Bhatt NR, Browne C, MacCraith E et al. (2016) Incidence, Cost, Complications and Clinical Outcomes of Iatrogenic Urethral Catheterization Injuries: A Prospective Multi-Institutional Study. J Urol 196: 1473-1477. [Crossref]
- Santucci RA, Joyce GF, Wise M (2007) Male urethral stricture disease. J Urol 177: 1667-1674. [Crossref]
- McAninch JW (2005) Urethral reconstruction: a continuing challenge. J Urol 173: 7. [Crossref]
- Mundy AR (1995) The long-term results of skin inlay urethroplasty. Br J Urol 75: 59-61. [Crossref]
- Dublin N, Stewart LH (2004) Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int 94: 867-869. [Crossref]
- Barbagli G, Palminteri E, De Stefani S, Lazzeri M (2006) Penile urethroplasty: techniques and outcomes using buccal mucosa grafts. Contemp Urol 18: 25-33.
- Chung YG, Duong Tu, Debra Franck, Eun Seok Gil, Khalid Algarrahi et al. (2014) Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PloS one 9: e91592. [Crossref]
- Jia W, Tang H, Wu J, Hou X, Chen B et al. (2015) Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 69: 45-55. [Crossref]
- Pinnagoda K, Larsson HM, Vythilingam G, Vardar E, Engelhardt EM et al. (2016) Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater 43: 208-217. [Crossref]
- Atala A, Danilevskiy M, Lyundup A, Glybochko P, Butnaru D et al. (2017) The potential role of tissue-engineered urethral substitution: clinical and preclinical studies. J Tissue Eng Regen Med 11: 3-19. [Crossref]
- Chun SY, Kim BS, Kwon SY, Park SI, Song PH et al. (2015) Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J Korean Med Sci 30: 301-307. [Crossref]
- Li C, Xu YM, Liu ZS, Li HB (2013) Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 81: 1075-1080. [Crossref]
- Rogovaya OS, Fayzulin AK, Vasiliev AV, Kononov AV, Terskikh VV (2015) Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta naturae 7: 70-77. [Crossref]
- Sartoneva R, Haaparanta AM, Lahdes-Vasama T, Mannerström B, Kellomäki M et al. (2012) Characterizing and optimizing poly-L-lactide-co-epsilon-caprolactone membranes for urothelial tissue engineering. J R Soc Interface 9: 3444-3454. [Crossref]
- Sun D, Yang Y, Wei Z, Xu Y, Zhang X et al. (2014) Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med 18: 434-443. [Crossref]
- Wu S, Cheng Z, Liu G, Zhao X, Zhong L et al. (2013) Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol 36: 63-69. [Crossref]
- Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A (2008) Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol 26: 323-326. [Crossref]
- Zhang Y, McNeill E, Tian H, Soker S, Andersson KE et al. (2008) Urine derived cells are a potential source for urological tissue reconstruction. J Urol 180: 2226-2233. [Crossref]
- Bharadwaj S, Liu G, Shi Y, Markert C, Andersson KE et al. (2011) Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng A 17: 2123-2132. [Crossref]
- Versteegden LRM, de Jonge PKJD, IntHout J, van Kuppevelt TH, Oosterwijk E et al. (2017) Tissue Engineering of the Urethra: A Systematic Review and Meta-analysis of Preclinical and Clinical Studies. Eur Urol 72: 594-606. [Crossref]
- Engel O (2012) Tissue-engineered buccal mucosa urethroplasty: Outcome of our first 10 patients. J Urol 187: e6.
- Fossum M, Skikuniene J, Orrego A, Nordenskjold A (2012) Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr 101: 755-760. [Crossref]
- Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR (2008) Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol 53: 1263-1269. [Crossref]
- Osman NI, Patterson JM, MacNeil S, Chapple CR (2014) Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol 66: 790-791. [Crossref]
- Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S et al. (2011) Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377: 1175-1182. [Crossref]
- Ramsay S, Ringuette-Goulet C, Langlois A, Bolduc S (2016) Clinical challenges in tissue-engineered urethral reconstruction. Transl Androl Urol 5: 267-270. [Crossref]
- Aittomaki K, Eroila H, Kajanoja P (2001) A population-based study of the incidence of Mullerian aplasia in Finland. Fertil Steril 76: 624-625. [Crossref]
- Oppelt P, Renner SP, Kellermann A, Brucker S, Hauser GA et al. (2006) Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod 21: 792-797. [Crossref]
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89: 501-506. [Crossref]
- Rogers GR, Villarreal A, Kammerer-Doak D, Qualls C (2001) Sexual function in women with and without urinary incontinence and/or pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 12: 361-365. [Crossref]
- Health (2011) U.S.F.a.D.A.C.f.D.a.R. Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse.
- Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E et al. (2001) Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 98: 646-651. [Crossref]
- Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol 188: 108-115. [Crossref]
- Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S et al. (2002) Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol 186: 712-716. [Crossref]
- Weber AM, Richter HE (2005) Pelvic organ prolapse. Obstet Gynecol 106: 615-634. [Crossref]
- Kurt S, Canda MT, Tasyurt A (2013) A new surgical method of suprapubic and extraperitoneal approach with uterine preservation for pelvic organ prolapse: kurt extraperitoneal ligamentopexy. ISRN Obstet Gynecol 2013: 748232. [Crossref]
- Smith FJ, Holman CD, Moorin RE, Tsokos N (2010) Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol 116: 1096-1100. [Crossref]
- Maher CM, Feiner B, Baessler K, Glazener CM (2011) Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J 22: 1445-1457. [Crossref]
- Blandon RE, Gebhart JB, Trabuco EC, Klingele CJ (2009) Complications from vaginally placed mesh in pelvic reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct 20: 523-531. [Crossref]
- Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol 113: 367-373. [Crossref]
- Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S (2008) Surgical management of pelvic organ prolapse in women: a short version Cochrane review. Neurourol Urodyn 27: 3-12. [Crossref]
- Sung VW, Rogers RG, Schaffer JI, Balk EM, Uhlig K et al. (2008) Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol 112: 1131-1142. [Crossref]
- Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL et al. (2011) Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J 22: 789-798. [Crossref]
- Administration U.S.F.a.D (2019) FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices.
- Amankwah YA, Haefner HK, Brincat CA (2010) Management of vulvovaginal strictures/shortened vagina. Clin Obstet Gynecol 53: 125-133. [Crossref]
- O'Boyle AL, O'Boyle JD, Calhoun B, Davis GD (2005) Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct 16: 69-72. [Crossref]
- DeLancey JO, Kearney R, Chou Q, Speights S, Binno S (2003) The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 101: 46-53. [Crossref]
- DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K et al. (2007) Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 109: 295-302. [Crossref]
- Wefer J, Sekido N, Sievert KD, Schlote N, Nunes L et al. (2002) Homologous acellular matrix graft for vaginal repair in rats: a pilot study for a new reconstructive approach. World J Urol 20: 260-263. [Crossref]
- Morton KE, Davies D, Dewhurst J (1986) The use of the fasciocutaneous flap in vaginal reconstruction. Br J Obstet Gynaecol 93: 970-973. [Crossref]
- Hendren WH, Atala A (1994) Use of bowel for vaginal reconstruction. J Urol 152: 752-755. [Crossref]
- Wiser WL, Bates GW (1984) Management of agenesis of the vagina. Surg Gynecol Obstet 159: 108-112. [Crossref]
- Morton KE, Dewhurst CJ (1986) Human amnion in the treatment of vaginal malformations. Br J Obstet Gynaecol 93: 50-54. [Crossref]
- Zhou JH, Sun J, Yang CB, Xie ZW, Shao WQ et al. (2010) Long-term outcomes of transvestibular vaginoplasty with pelvic peritoneum in 182 patients with Rokitansky's syndrome. Fertil Steril 94: 2281-2285. [Crossref]
- Ding JX, Zhang XY, Chen LM, Hua KQ (2013) Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-von-Rokitansky-Kuster-Hauser syndrome: a prospective new technique for vaginal reconstruction. Gynecol Obstet Invest 75: 93-96. [Crossref]
- Lin WC, Chang CY, Shen YY, Tsai HD (2003) Use of autologous buccal mucosa for vaginoplasty: a study of eight cases. Hum Reprod 18: 604-607. [Crossref]
- Li FY, Xu YS, Zhou CD, Zhou Y, Li SK et al. (2014) Long-term outcomes of vaginoplasty with autologous buccal micromucosa. Obstet Gynecol 123: 951-956. [Crossref]
- De Filippo RE, Yoo JJ, Atala A (2003) Engineering of vaginal tissue in vivo. Tissue Eng 9: 301-306. [Crossref]
- De Filippo RE, Bishop CE, Filho LF, Yoo JJ, Atala A (2008) Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 86: 208-214. [Crossref]
- Panici PB, Bellati F, Boni T, Francescangeli F, Frati L et al. (2007) Vaginoplasty using autologous in vitro cultured vaginal tissue in a patient with Mayer-von-Rokitansky-Kuster-Hauser syndrome. Hum Reprod 22: 2025-2028. [Crossref]
- Nodale C, Vescarelli E, D'Amici S, Maffucci D, Ceccarelli S et al. (2014) Characterization of human vaginal mucosa cells for autologous in vitro cultured vaginal tissue transplantation in patients with MRKH syndrome. Biomed Res Int 2014: 201518. [Crossref]
- Zhang JK, Du RX, Zhang L, Li YN, Zhang ML et al. (2017) A new material for tissue engineered vagina reconstruction: Acellular porcine vagina matrix. J Biomed Mater Res A 105: 1949-1959. [Crossref]
- Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P et al. (2014) Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384: 329-336. [Crossref]
- Auger FA, Berthod F, Moulin V, Pouliot R, Germain L (2004) Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem 39: 263-275. [Crossref]
- Magnan M, Lévesque P, Gauvin R, Dubé J, Barrieras D et al. (2009) Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng Part A 15: 197-202. [Crossref]
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA (1998) A completely biological tissue-engineered human blood vessel. FASEB J 12: 47-56. [Crossref]
- Michel M, L'Heureux N, Pouliot R, Xu W, Auger FA et al. (1999) Characterization of a new tissue-engineered human skin equivalent with hair. In vitro cellular & developmental biology. Animal 35: 318-326. [Crossref]
- Vermette M, Trottier V, Ménard V, Saint-Pierre L, Roy A et al. (2007) Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 28: 2850-2860. [Crossref]
- Bouhout S, Perron E, Gauvin R, Bernard G, Ouellet G et al. (2010) In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng Part A 16: 1539-1548. [Crossref]
- Orabi H, Saba I, Rousseau A, Bolduc S (2017) Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl Res 180: 22-36. [Crossref]
- Cattan V, Bernard G, Rousseau A, Bouhout S, Chabaud S et al. (2011) Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol 60: 1291-1298. [Crossref]
- Bouhout S, Chabaud S, Bolduc S (2016) Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J Urol 34: 121-130. [Crossref]
- Rousseau A, Fradette J, Bernard G, Gauvin R, Laterreur V et al. (2015) Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. J Tissue Eng Regen Med 9: E135-E143. [Crossref]
- Li H, Xu Y, Xie H, Li C, Song L et al. (2014) Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A 20: 774-784. [Crossref]
- Wang Y, Fu Q, Zhao RY, Deng CL (2014) Muscular tubes of urethra engineered from adipose-derived stem cells and polyglycolic acid mesh in a bioreactor. Biotechnol Lett 36: 1909-1916. [Crossref]
- Zhang M, Xu MX, Zhou Z, Zhang K, Zhou J et al. (2014) The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PloS one 9: e95583. [Crossref]
- Kang HS, Choi SH, Kim BS, Choi JY, Park GB et al. (2015) Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J Korean Med Sci 30: 1764-1776. [Crossref]
- Aubin K, Vincent C, Proulx M, Mayrand D, Fradette J (2015) Creating capillary networks within human engineered tissues: impact of adipocytes and their secretory products. Acta biomater 11: 333-345. [Crossref]
- Imbeault A, Bernard G, Rousseau A, Morissette A, Chabaud S et al. (2013) An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can Urol Assoc J 7: E4-E9. [Crossref]
- Chabaud S, Rousseau A, Marcoux TL, Bolduc S (2015) Inexpensive production of near-native engineered stromas. J Tissue Eng Regen Med.
- Hansbrough JF (1990) Current status of skin replacements for coverage of extensive burn wounds. J Trauma 30: S155-S160. [Crossref]
- Krejci NC, Cuono CB, Langdon RC, McGuire J (1991) In vitro reconstitution of skin: fibroblasts facilitate keratinocyte growth and differentiation on acellular reticular dermis. J Invest Dermatol 97: 843-848. [Crossref]
- Young DM, Greulich KM, Weier HG (1996) Species-specific in situ hybridization with fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic mice. J Burn Care Rehabil 17: 305-310. [Crossref]
- Converse JM, Ballantyne DL Jr (1962) Distribution of diphosphopyridine nucleotide diaphorase in rat skin autografts and homografts. Plast Reconstr Surg Transplant Bull 30: 415-425. [Crossref]
- Haller JA Jr, Billingham RE (1967) Studies of the origin of the vasculature in free skin grafts. Ann Surg 166: 896-901. [Crossref]
- Lambert PB (1971) Vascularization of skin grafts. Nature 232: 279-280. [Crossref]
- Demarchez M, Hartmann DJ, Prunieras M (1987) An immunohistological study of the revascularization process in human skin transplanted onto the nude mouse. Transplantation 43: 896-903. [Crossref]
- Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA (2005) Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant 5: 1002-1010. [Crossref]
- Vanni AJ (2015) New frontiers in urethral reconstruction: injectables and alternative grafts. Transl Androl Urol 4: 84-91. [Crossref]
- Yang SX, Yao Y, Hu YF, Song C, Wang LL et al. (2004) Reconstruction of rabbit urethra using urethral extracellular matrix. 117: 1786-1790. [Crossref]
- Sievert KD, Wefer J, Bakircioglu ME, Nunes L, Dahiya R et al. (2001) Heterologous acellular matrix graft for reconstruction of the rabbit urethra: histological and functional evaluation. J Urol 165: 2096-2102. [Crossref]
- Morissette A IA, Cattan V, Bernard G, Taillon G, Chabaud S et al. (2013) Strategies to Reconstruct a Functional Urethral Substitute by Self-Assembly Method. Procedia Engineer 59: 8.
- Kumar D, Anand T, Kues WA (2017) Clinical potential of human-induced pluripotent stem cells: Perspectives of induced pluripotent stem cells. Cell Biol Toxicol 33: 99-112. [Crossref]
