Journals
Successful Desensitization to fNIRS Cap of a Child with ASD: Case-Study
A B S T R A C T
Advances in neuroimaging technology allows for the investigation of neural activity patterns of many different populations. Some populations, such as individuals with Autism Spectrum Disorders (ASD) present with particular challenges, such as hypersensitivities to touch especially around the head and face. Many studies report therefore having to exclude participants due to inability to comply with equipment requirements. The purpose of this particular study was to systematically desensitize a child with ASD to a 44-channel functional Near-Infrared Spectroscopy cap.
The participant was a 10-year-old child with reported hypersensitivities to touch around the head. A systematic desensitization procedure was employed including a Brief Multiple-Stimulus-Without Replacement (MSWO) preference assessment, a token economy, as well as gradual increase of optodes to the cap. Results indicate that the participant was able to increase tolerance of the cap with each successive desensitization sessions, as well as completing the experimental task.
The results from this case-study indicate that a systematic approach can be employed to successfully desensitize a child with ASD to wear the fNIRS cap to successfully complete an experimental task. Future research should expand the systematic approach to more participants with varying severity levels of ASD.
K E Y W O R D S
Autism spectrum disorders, near-infrared spectroscopy, desensitization, applied behavior analysis.
Case Study
Neuroimaging techniques are advancing rapidly in precision and functionality. With such advances, we have been able to learn many things about structural and functional differences of the brain between typical individuals and individuals with disabilities or disorders. Neuroimaging has given us some insight into the brain mechanisms of individuals with Autism Spectrum Disorders (ASD). Thus far, we have established differences in activation patterns in specific regions as well as atypical inter- and intrahemispheric connectivity [1-9]. Further, studies have found structural differences due to overgrowth and undergrowth of certain regions during certain ages [10-12]. Technologies such as Positron Emission Tomography (PET), Diffusion Tensor Imaging (DTI), and Magnetic Resonance Spectroscopy (MRS) have been used to conduct neuroimaging studies. More commonly though, functional Magnetic Resonance Imaging (fMRI), Electroencephalogram (EEG) and recently functional Near-Infrared Spectroscopy (fNIRS) are dominant in neuroimaging studies with individuals with ASD. Each technology presents advantages and disadvantages. Comparatively, there are few studies with individuals with ASD, especially children, due to challenges in compliance and tolerance of the different types of equipment. While fMRI imaging technology has the best spatial resolution at the level of voxel sizes, the fMRI imaging environment is not child-friendly, recording imaging data is susceptible to motion artifacts, the spatial confinement and noise level is challenging for some individuals with ASD and the analyses are complex. Kana, Libero, and Moore (2011) describe special techniques that were employed with individuals with developmental disabilities to ensure successful testing with fMRIs. They (1) used social stories, explaining the unusual environment and often confusing situation for children with ASD. Social stories have been shown to be effective in helping children adjust to unknown situations [13]. They (2) recorded scanner sounds to acclimate the individuals with ASD to the sounds that are produced by the scanner. (3) A discarded old mock-MRI scanner was used to acclimate participants to the environment. (4) A tour of the MRI scanner was provided prior to the scanning, to again familiarize the individual with the equipment as well as the upcoming procedures. Great measures were taken to (5) make the MRI scanner child-friendly by decorating it with stickers, providing blankets and making the scanner look like an item of interest (e.g., trains). Lastly, (6) the participants were given the option of watching cartoons or movies during the anatomical and DTI acquisition. Even though extensive preparatory measures were taken to make fMRI scanning most successful with this group of participants, motion artifacts, anxiety and refusal to enter the scanner were still a major concern and some data had to be excluded from the analysis due to the special considerations of this population.
Neuroimaging with EEG equipment allows for a more natural experimental environment in which the participant can be seated at a table, is not confined to a tube and noise is minimized. EEG has the potential to record neurological activity of verbal responses. Unfortunately, EEG data is susceptible to motion artifacts as little as the movement during a swallow. Many individuals with ASD report hypersensitivities which results in more movement and fidgeting. Baker, Lane, Angley, and Young (2008) reported that out of the 22 participants with ASD, 82% had some sort of sensory processing difficulty according to a parent questionnaire [14]. They further report the occurrence of children who exhibit both hypo- and hyper-responsiveness. Sensory-overresponsivity was found in the cluster of tactile sensitivity, movement sensitivity and visual/auditory sensitivity. Poor sensory processing was associated with higher levels of behavior and emotional problems. Baranek, Boyd, Poe, David, and Watson (2007) observed hyper-responsive sensory patterns to be characteristic of developmental delays in general, including those children with ASD [15].
A study conducted by Blakemore et al. (2006) confirmed hypersensitivity to tactile stimuli for those diagnosed with High-functioning autism [16]. Grooming and hygiene tasks posed a particular challenge in 60.9% of children with ASD (Tomchek & Dunn, 2007), indicating the head and facial area to be particularly sensitive [17]. These hypersensitivities pose a significant challenge in relation to EEG and fMRI neuroimaging, where children are required to lay still in small tubes for long periods of time, with packing around their heads and/or bodies or wearing an EEG cap. Similar to EEG, fNIRS is a technology that allows measurement of neurological activity during natural tasks, meaning that verbal responses are possible instead of button presses. It allows the participant to sit in a natural setting, while wearing a fNIRS cap. While fNIRS does not have a spatial resolution as accurate as a voxel size, it has temporal resolution of 1/10 sec, providing excellent temporal resolution. It measures oxygenated, deoxygenated and total blood concentration levels. Motion artifacts in the raw data is filtered through preprocessing, posing less of a challenge compared to EEG.
Even though motion artifacts and the confined space are not as concerning with fNIRS as they are with fMRI and EEG, wearing the fNIRS cap can still pose a challenge due to reported hypersensitivities. Multiple fNIRS studies report the exclusion of ASD participants due to intolerance of the fNIRS cap. Therefore, it is important to prepare children with ASD for the upcoming procedures. For the purpose of this case-study, the following research questions emerged: (1) Is there a difference in the average time children with and without ASD tolerate wearing the fNIRS cap during an in-vivo experimental task? (2) Would a child with ASD with reported hypersensitivities around the head be able to tolerate wearing the fNIRS cap for the duration of the experiment after systematic desensitization? Regarding the first research question, we anticipated that children with ASD would tolerate the fNIRS cap significantly less than typically developing children during an in-vivo task. Considering research question two, we hypothesized that a systematic sensory desensitization training would be helpful for getting a hypersensitive child with ASD to wear the NIRS caps. Sensory desensitization training has been shown to be successful in getting children with ASD to wear EEG caps. A gradual process was employed to approximate the end goal of wearing the net for 10 minutes. Ten out of the 12 participants completed the training successfully and were therefore able to finish the actual EEG tasks [18]. NIRS caps have a similar set‐up as an EEG net.
Methods
A larger fNIRS study involved measuring hemodynamic responses from children with and without ASD, ranging in ages 8 through 16 years old. Parents for each participant, regardless of autism diagnosis or neurotypical development, were interviewed about potential hypersensitivities to things worn on the head. Irrespective of reported hypersensitivities, each of the participants received a tour of the testing room, the instrumentation, and cap set-up. Further, participants received a child-appropriate explanation of the capping procedures, as well as the measures that were gathered. Out of a total of 30 participants (i.e. 20 typically developing children and 10 children with ASD), one mother of a child with ASD reported concerns of her child’s ability to wear the cap for the entirety of the session. This participant was a 10 year,10-month-old Caucasian male with a medical diagnosis of ASD. He had received Early Intensive Behavior Intervention (EIBI) from 3-6 years of age. At the time of the study, he attended a public school and received special education services. His nonverbal full-scale intelligence quotient (FSIQ) as measured by the Universal Nonverbal Intelligent Test (UNIT, citation) was 75, indicating a nonverbal intelligence below average. His verbal skills were sufficient to respond to simple questions with spoken words in full sentences. Since it was anticipated that some children, especially those with ASD, would need desensitization to the fNIRS cap, a systematic procedure was developed combing multiple methods from the field of Applied Behavior Analysis (ABA). The procedure was employed with the child with hypersensitivities to prevent exclusion from the study. The procedure included a Brief Multiple-Stimulus Without Replacement (Brief MSWO) preference assessment and a token economy system together with gradually increasing the time wearing the fNIRS cap while gradually adding more optodes to the cap [20, 21].
Brief MSWO
The Brief MSWO consisted of interviewing the mother again about potential toys that could function as a reinforcer for the child. The mother provided a list of toys (i.e., light up toys, squishy toys, cars, balls, etc.). Five of those toys were selected and placed in front of the child prior to each session. The toys were placed on a table at equal distance from each other in front of the child. The child was then instructed to “pick one”. The item, which was chosen first, was given the first rank for the first round. After the child had chosen one item, the remaining four items were rearranged and placed in front of the child again with the same succinct instruction “pick one”. The item that was picked this time was recorded as rank #2. This was repeated until all five items were gone. The entire procedure was completed three times for reliability regarding ranks. After three rounds of choosing toys, ranks were added to determine the most preferred items. The lowest sum (i.e., highest preferred item received rank #1) was determined to be the highest preferred item and was used as the reinforcer for the session. During each desensitization session and subsequent experimental fNIRS sessions, the reinforcer was placed visibly in front of the child and out of reach.
Token Economy
In addition to identifying an item that functioned as a reinforcer, we introduced a token economy system [22, 23]. According to the mother, the child was familiar with token economy systems from prior EIBI. The token economy consisted of a simple sheet of paper with five boxes that was placed in front of the child. A box was checked by the experimenter after a time period had elapsed. Time periods changed depending on the progression towards tolerating the fNIRS cap. The time period was determined prior to the beginning of each desensitization session. Initially, each time period was 120 seconds long for wearing the cap without any optodes. With increased optodes in the cap the length initially decreased to 60 seconds intervals before returning to 120 second intervals. A timer was placed in front of the child to provide feedback for the child and to track the time accurately. When all ten boxes were checked, the fNIRS cap was taken off the child and the reinforcer was delivered.
Figure 1: Optode set up.
fNIRS Cap Setup
Initially, the fNIRS cap was placed on the childs’ head without any optodes. The cap is made out of elastic neoprene material that sits tight on the head, like a swim cap. It has 44 holes for each optode. This particular fNIRS cap was designed to place optodes over the frontal lobe and left lateral lobes, to ensure hemodynamic responses of four regions of interests (i.e., superior temporal cortex, inferior parietal lobule, dorsolateral prefrontal cortex, and orbital frontal cortex) were measured. Criteria was set to wearing the cap for 10 minutes. Once the child tolerated the cap without optodes for 10 minutes, optodes were added to the cap as well as decreasing the interval time length on the token economy. Figure 1 displays the cap with all optodes in place. Once the child tolerated the fNIRS cap for 10 minutes with half of the optodes, the complete set of optodes was placed in the cap. After meeting criterion of 10 minutes with all optodes, the child was prepared to commence with the actual experimental task.
Procedure
During each desensitization session, the child was seated in a chair facing a computer screen on a desk to familiarize him with the actual experimental session. The experimenter was seated next to the child, engaging the child in simple conversation through questions about preferred topics (e.g., computer games). This setup approximated the actual fNIRS session as closely as possible. The arrangement was consistent throughout all sessions. During the first session, the cap was placed on the child’s head for the first 10 minutes without any optodes while checking a box every 120 seconds of tolerating the cap. After meeting criteria, half of the optodes were placed into the cap and the time to earn a check mark was lowered to 60 seconds. Again, after meeting criteria, the interval length was increased to 120 seconds with half of the optodes in place. Onc the child tolerated half of the optodes for at least a total of 10 minutes, the remainder of the optodes was added to the cap and placed on the child’s head. A checkmark was earned after 60 seconds of wearing the cap for an entire minute. During the final portion of desensitization, the child wore the cap for a total of 10 minutes with all optodes in place. This time period was the approximate time that was previously determined to be necessary to answer at least 30 items on the experimental task. We therefore determined that the child was sufficiently prepared for participating in the actual fNIRS tasks.
Table 1: Time Under the Cap in Minutes Displayed as Decimals
|
Time under cap |
TD Match |
Time under cap |
|
|
A06 |
14:50 |
C33 |
25:58 |
|
|
C24 |
20:01 |
|
|
A08 |
26:38 |
C23 |
N/A |
|
|
C20 |
29:52 |
|
|
A09 |
26:02 |
C18 |
20:36 |
|
|
C27 |
19:59 |
|
|
A11 |
25:17 |
C35 |
20:48 |
|
|
C19 |
21:10 |
|
|
A12 |
10:29 |
C37 |
19:50 |
|
|
C31 |
22:41 |
|
|
A13 |
13:22 |
C40 |
16:25 |
|
|
C38 |
16:46 |
|
|
A14 |
12:01 |
C16 |
22:32 |
|
|
C17 |
26:01 |
|
|
A25 |
22:07 |
C32 |
22:45 |
|
|
C39 |
14:15 |
|
|
A26 |
16:05 |
C15 |
19:43 |
|
|
C30 |
21:46 |
|
|
A28 |
26:32 |
C29 |
22:09 |
|
|
C22 |
26:43 |
Results
Research Question 1
Time under the fNIRS cap was recorded for all 30 participants regardless of prior desensitization to the cap. All children in both groups were able to wear the fNIRS caps while they responded to a minimum of 25 experimental items. Children in the ASD group had a duration under the cap that ranged from 10.48 to 26.63 minutes with a mean of 19.51 minutes and a standard deviation of 6.48 minutes. The range of duration under the cap for the TD group was 14.25 to 29.87 minutes, with a mean of 21.57 minutes and a standard deviation of 3.76 minutes. The length of time that the fNIRS cap was tolerated during the experimental procedure did not differ significantly for the two groups, t(12.289) = ‐.93, p = .37, d= .39. See Table 1 for minutes under the cap for each participant.
Figure 2: Desensitization progression.
Research Question 2
One child in the ASD group required desensitization training in order to tolerate the fNIRS cap with all optodes during the experimental procedure. The progression of cap tolerance during training is displayed in Figure 2. During the first desensitization session, this child was able to wear the cap without optodes for 10 minutes. During the next session, he tolerated wearing the cap for 5 minutes with half of the optodes in place, which was then increased to 10 minutes during the next session. The following session he was able to wear the cap for 5 minutes with all optodes in place. During the last desensitization session, he wore the cap for 10 minutes with all optodes in place. He was able to increase cap tolerance to a total of 14.83 minutes during the experiment. These results suggested that both children with and without ASD in the age range of 9‐15 years are able to tolerate wearing the cap for approximately 15 minutes after careful and systematic desensitization and by utilizing behavioral strategies throughout the testing session. While children with ASD wore the cap with slightly lesser duration, they were able to successfully participate in the study.
Discussion
The larger fNIRS study assessed the neural activation patterns of children with and without ASD while they answered questions about social situations. Neuroimaging was conducted in-vivo, enabling functional imaging as children listened to questions, thought about their answers, and responded verbally. We used fNIRS to record oxygenated (HbO), deoxygenated (HbR), and total (HbT) hemoglobin concentration values at 100 msec intervals. Based on previous neuroimaging research, we had identified four ROIs that were likely to play a role in the responses to pragmatic language tasks. Those regions were Dorsolateral Prefrontal Cortex (DLPFC) left, Orbitofrontal Cortex (OFC), Superior-Temporal Gyrus (STG) and Inferior Parietal Lobule (IPL).
Previous research on the neural activation used equipment such as PET, EEG and fMRI, Unfortunately, constraints inherent in the previously mentioned imaging procedures interfere with the ability to record neural data while participants are engaged in verbal tasks. Neural images from these systems are obtained as participants press a button and/or think about an answer to a question. Further, there are no neuroimaging studies on young children with ASD; imaging research with individuals with ASD has been limited to the study of adolescents or adults with ASD. Three important reasons for the lack of imaging research with younger individuals with ASD are the expense of imaging, the necessity for participants to lie in a confined space for an extended period of time and the need for participants to be very still during the entire imaging process. fNIRS counters all these disadvantages because it is relatively inexpensive, it can be conducted in a regular room, and data processing and filtering algorithms account for motion artifacts in fNIRS compared to other neuroimaging equipment [24]. Children can sit at a table and can interact directly with another person while being imaged. In this study, children with ASD and their TD controls wore optode caps that captured hemodynamic responses from the frontal lobe and left hemisphere as they responded to questions about a social situation. During a neuroimaging session with fNIRS, the optode cap must sit tightly on the head of the participant. This is necessary to emit and receive the proper signal from the NIR optodes. Hypersensitivities to touch would make it difficult for children to tolerate wearing the cap for long periods, which would interfere with the ability to collect useful data. Therefore, we had to ensure that all participants would tolerate the optode cap for the duration of the task.
Early in the description of autism, Dr. Asperger described hypersensitivities of senses, especially touch, smell and taste [16, 25]. A number of techniques have been developed to decrease hypersensitivities for different senses, and to eliminate phobias and anxiety. Desensitization training refers to a procedure in which the problematic situation is analyzed into its different components. This task analysis includes environmental conditions, personnel, and a step-by-step outline of the task. The situation is then recreated with one component added at a time. Mastery at each step has to be established before moving to the next step. Desensitization has been shown to be successful for children with ASD for managing dental visits, auditory stimuli, and different types of phobias [26-31].
In this study, all parents were interviewed prior to the experiment regarding potential hypersensitivities. One mother of a child with ASD reported her child to be sensitive to touch, especially to the head. None of the mothers of children in the TD group reported that their children had hypersensitivities to touch. Development of the desensitization procedure required a detailed task analysis. Brief Multiple-Stimulus Without Replacement Preference Assessment (Brief-MSWO) prior to each session was combined with systematic desensitization. The child identified a highly preferred item, which was then used as a reinforcer. A token economy system with timed intervals was used throughout. Each time-interval wearing the cap earned the child a token. At the beginning of the training the child tried to remove the cap from his head. The experimenter prevented this and reminded the child about the token system used for reinforcement. While the child was wearing the cap, he was engaged in a conversation with the experimenter. After mastery of one step, more optodes were added to the cap. At each new level the child initially voiced discomfort. The frequency of those utterances decreased with the progression of training. Finally, the child was able to wear the cap during the experiment and completed all the requirements.
The individualized desensitization was successful for this child. This meant that the child did not remove the cap from his head during the experiment. While he touched the optodes occasionally, he did not remove the cap, allowing for proper scanning to occur. During the actual experiment he wore the cap for a total of 14:50 min, which was even longer then he was trained for. The chosen highly preferred item was used as a reinforcer for earning five tokens. Throughout the sessions, the experimenter asked the children if they were comfortable about every five trials. When a child indicated discomfort, a short rest period was initiated, and the cap was removed following the resting state. Children from both groups wore the cap between 10 and 29 minutes, with an average of 20 minutes. Findings suggested that children with and without ASD between the 9 and 15 years can participate in fNIRS experiments that last between 10 and 20 minutes. These time frames need to be taken into consideration when planning new studies.
Finding particularly from the case-study indicate that a child with hypersensitivities can be systematically trained and desensitized to tolerate wearing the optode cap for the duration of a short experimental fNIRS task. However, it may be unusual to have a sample of children with ASD in which only one individual displays hypersensitivity. This might be due to the fact that we recruited higher-functioning children with ASD who had to display sufficient verbal skills to complete the experimental task. This study is limited in that the children in this study had relatively small amounts of hypersensitivity to our optode caps. Development of more robust desensitization procedures would require a larger number of individuals showing hypersensitivities. Differing patterns of behavior in children with ASD may necessitate different desensitization procedures than the ones used here. However, this case-study provides guidance for use in other fNIRS studies.
Future research should focus on refining procedures to enable inclusion of younger children with ASD who may exhibit more stereotypies and challenging behavior. With the inclusion of more and younger children, studies should also focus on including children with less verbal skills to increase understanding of underlying neurological processes. Future studies should also narrow the age ranges, as well as language abilities and stereotypic behavior. Benefits include greater understanding of the relationships between neurological activation patterns and pragmatic language, the development of effective desensitization procedures and potential effects of different types of interventions.
Article Info
Article Type
Case StudyPublication history
Received: Fri 26, Apr 2019Accepted: Sat 11, May 2019
Published: Thu 30, May 2019
Copyright
© 2023 Daphne Hartzheim. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2019.02.01
Author Info
Daphne Hartzheim Ronald B. Gillam
Corresponding Author
Daphne HartzheimLouisiana State University, Department of Communication Sciences and Disorders, 86 Hatcher Hall, Baton Rouge, LA 70803
Figures & Tables

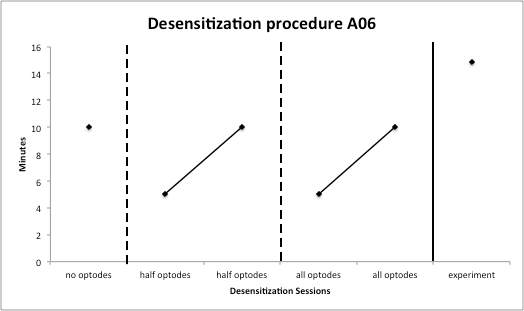
Table 1: Time Under the Cap in Minutes Displayed as Decimals
|
Time under cap |
TD Match |
Time under cap |
|
|
A06 |
14:50 |
C33 |
25:58 |
|
|
C24 |
20:01 |
|
|
A08 |
26:38 |
C23 |
N/A |
|
|
C20 |
29:52 |
|
|
A09 |
26:02 |
C18 |
20:36 |
|
|
C27 |
19:59 |
|
|
A11 |
25:17 |
C35 |
20:48 |
|
|
C19 |
21:10 |
|
|
A12 |
10:29 |
C37 |
19:50 |
|
|
C31 |
22:41 |
|
|
A13 |
13:22 |
C40 |
16:25 |
|
|
C38 |
16:46 |
|
|
A14 |
12:01 |
C16 |
22:32 |
|
|
C17 |
26:01 |
|
|
A25 |
22:07 |
C32 |
22:45 |
|
|
C39 |
14:15 |
|
|
A26 |
16:05 |
C15 |
19:43 |
|
|
C30 |
21:46 |
|
|
A28 |
26:32 |
C29 |
22:09 |
|
|
C22 |
26:43 |
References
- Catani M, Dell’Acqua F, Budisavljevic S, Howells H, Thiebaut de Schotten M et al. (2016) Frontal networks in adults with autism spectrum disorder. Brain 139: 616-630. [Crossref]
- Delmonte S, Gallagher L, O'hanlon E, McGrath J, Balsters JH (2013) Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci 7: 430. [Crossref]
- Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA (2013) Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci 7: 444. [Crossref]
- Kikuchi M, Shitamichi K, Yoshimura Y, Ueno S, Hiraishi H et al. (2013) Altered brain connectivity in 3-to 7-year-old children with autism spectrum disorder. Neuroimage Clin 2: 394-401. [Crossref]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M et al. (2009) Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage 47: 764-772. [Crossref]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM et al. (2011) Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb cortex 21: 2233-2243. [Crossref]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL et al. (2011) Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry 70: 270-277. [Crossref]
- Shih P, Shen M, Öttl B, Keehn B, Gaffrey MS et al. (2010) Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia 48: 2931-2939. [Crossref]
- Zhu H, Fan Y, Guo H, Huang D, He S (2014) Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomed Opt Express 5: 1262-1274. [Crossref]
- Ander BP, Barger N, Stamova B, Sharp FR, Schumann CM (2015) Atypical miRNA expression in temporal cortex associated with dysregulation of immune, cell cycle, and other pathways in autism spectrum disorders. Mol Autism 6: 37. [Crossref]
- Courchesne E, Campbell K, Solso S (2011) Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 1380: 138-145. [Crossref]
- Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C et al. (2015) Different functional neural substrates for good and poor language outcome in autism. Neuron 86: 567-577. [Crossref]
- Thiemann KS, Goldstein H (2001) Social stories, written text cues, and video feedback: Effects on social communication of children with autism. J Appl Behav Anal 34: 425-446. [Crossref]
- Baker AE, Lane A, Angley MT, Young RL (2008) The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: a pilot study. J Autism Dev Disord 38: 867-875. [Crossref]
- Baranek GT, Boyd BA, Poe MD, David FJ, Watson LR (2007) Hyperresponsive sensory patterns in young children with autism, developmental delay, and typical development. Am J Ment Retard 112: 233-245. [Crossref]
- Tomchek SD, Dunn W (2007) Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther 61: 190-200. [Crossref]
- Li Y, Yu D (2016) Weak network efficiency in young children with Autism Spectrum Disorder: Evidence from a functional near-infrared spectroscopy study. Brain cogn 108: 47-55. [Crossref]
- Roesler CP, Flax J, MacRoy-Higgins M, Fermano Z, Morgan-Byrne J et al. (2013) Sensory desensitization training for successful net application and EEG/ERP acquisition in difficult to test children. Commun Disord Q 35: 14-20.
- Higbee TS, Carr JE, Harrison CD (2000) Further evaluation of the multiple-stimulus preference assessment. Res Dev Disabil 21: 61-73. [Crossref]
- Cooper JO, Heron TE, Heward WL (2007) Applied behavior analysis.
- Mangus B, Henderson H, French R (1986) Implementation of a token economy by peer tutors to increase on-task physical activity time of autistic children. Percept Mot Skills 63: 97-98. [Crossref]
- Tarbox RS, Ghezzi PM, Wilson G (2006) The effects of token reinforcement on attending in a young child with autism. Behavioral Interventions: Theory & Practice in Residential & Community‐Based Clinical Programs, 21: 155-164.
- Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P et al. (2014) Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage 1: 181-191. [Crossref]
- Asperger H (1944) Die “Autistischen Psychopathen” im Kindesalter. Eur Arch Psy Clin Neuroscience 117: 76-136.
- Blakemore SJ, Tavassoli T, Calò S, Thomas RM, Catmur C et al. (2006) Tactile sensitivity in Asperger syndrome. Brain Cogn 61: 5-13. [Crossref]
- Klein U, Nowak A (1998) Autistic disorder: a review for the pediatric dentist. Pediatr dent 20: 312-317. [Crossref]
- Luscre DM, Center DB (1996) Procedures for reducing dental fear in children with autism. J Autism Dev Disord 26: 547-556. [Crossref]
- Koegel RL, Openden D, Koegel LK (2004) A systematic desensitization paradigm to treat hypersensitivity to auditory stimuli in children with autism in family contexts. Res Prac Person Sever Disabil 29: 122-134.
- Luiselli JK (1978) Treatment of an autistic child's fear of riding a school bus through exposure and reinforcement. J Behav Ther Exp Psychiatry 9: 169-172.
- Rapp JT, Vollmer TR, Hovanetz AN (2005) Evaluation and treatment of swimming pool avoidance exhibited by an adolescent girl with autism. Behav Ther 36: 101-105.
- Shabani DB, Fisher WW (2006) Stimulus fading and differential reinforcement for the treatment of needle phobia in a youth with autism. J Appl Behav Anal 39: 449-452. [Crossref]
