Stimulation of Vagus By Phenylephrine Increases the Efficiency and Safety of Antidepressants and Anti-Epileptics
A B S T R A C T
Background: Earlier, we discovered the possibility of potentiation of the therapeutic effects of small (threshold) doses of CNS agents by phenylephrine and adrenaline, while eliminating their side effects. However, the question of the possibility of potentiation by phenylephrine and other CNS potentiators of high therapeutic doses of CNS agents remained unstudied. This study is devoted to the research of this problem.
Objective: The aim of the study was to investigate the effect of the threshold dose of phenylephrine on the antidepressant effect of amitriptyline and the anticonvulsant effect of diazepam, as well as their side sedation in high doses.
Method: The experiments were carried out on the animated models of depression (Porsolt test) and epilepsy (clonic pentylenetetrazole (PTZ)-induced seizures), resistant to antidepressants and antiepileptics even at high therapeutic doses. Side sedative effect of substances was evaluated in the "open field" test.
Results: We established that the stimulation of gastric vagal afferents with phenylephrine, when administered orally at threshold doses, potentiates the anticonvulsant effect of diazepam and the antidepressant effect of amitriptyline in high therapeutic doses to the maximum level that is impossible in their application by themselves, and at the same time eliminates their side sedation.
Conclusion: A synergistic effect of phenylephrine and CNS drugs on the peripheral and central links of the vagal stress-protective reflex is discussed. It is assumed that the potentiation of therapeutic effect by phenylephrine and the elimination of side effects of the CNS agents occurs as a result of strengthening the vagal stress-protective reflex, eliminating the drug stress.
Keywords
Phenylephrine, diazepam, amitriptyline, vagal stress-protective reflex, afferents
Introduction
In our experiments, it was shown that stimulation of vagal afferents of the gastric mucosa by adrenomimetics (adrenaline, phenylephrine) when administered systemically at threshold doses potentiates the therapeutic effect of small doses of analgesics, antidepressants, neuroleptics, antiepileptics, and other CNS drugs to the level of therapeutic effects of therapeutic doses of the CNS drugs, but does not enhance their side effects [1, 4].
This method is suitable for the safe treatment of moderate severe forms of epilepsy, depression, medium-intensity pain, not resistant to drugs, in the treatment of which good results are achieved when using CNS agents alone in high therapeutic doses, which cause undesirable but tolerable side effects for most patients. However, the potentiation of the effects of small doses of the CNS drugs does not yet allow us to obtain the maximum possible therapeutic effects, so this technology can hardly be successfully used to treat severe, resistant to the action of drugs of CNS diseases. In this connection, there was a question: is it possible to use the method of selective chemoreceptor stimulation of vagal afferents to potentiate not small, but high doses of the CNS drugs to obtain the maximum possible therapeutic effect? It is very important to find a method of treatment for severe forms of depression and epilepsy, resistant to the action of even the highest medicinal doses. To solve this problem, we conducted experiments on animal models of depression and epilepsy, resistant to antidepressants and antiepileptics, even at high therapeutic doses.
In our previous experiments, the high efficiency and safety of the systemic use of adrenaline as a stimulator of chemoreceptors of vagal afferents was shown to potentiate the therapeutic effects of analgesics, antidepressants and anticonvulsants [2-4]. A serious disadvantage of adrenaline is the impossibility of its oral administration due to rapid destruction in the digestive tract. At the same time, adrenomimetic phenylephrine, which as stimulant of vagal afferents after systemic administration is not inferior in activity to adrenaline, is allowed for oral administration, since does not break down in the digestive tract. Since the oral route of administration of drugs is the main one in the treatment of chronic CNS diseases, we used adrenomimetic phenylephrine as stimulator of chemoreceptors of vagal afferents, which, in contrast to adrenaline, can be administered either injectively or orally. The advantage of phenylephrine is its lower toxicity compared with adrenaline and the slow development of addiction in chronic use.
We suggested that stimulation of vagal afferents of the gastric mucosa with phenylephrine in the threshold, in itself ineffective dose, will, on the one hand, potentiate the anticonvulsant effect of diazepam and the antidepressant effect of amitriptyline, and on the other hand eliminate their side sedative effects in high therapeutic doses. The aim of the study was to investigate the effect of the threshold dose of phenylephrine on the antidepressant effect of amitriptyline and the anticonvulsant effect of diazepam, as well as their side sedation in high doses.
Methods
The experiments were carried out on the animated models of depression (Porsolt test) and epilepsy (clonic pentylenetetrazole (PTZ)-induced seizures), resistant to antidepressants and antiepileptics even at high therapeutic doses. Side sedative effect of substances was evaluated in the "open field" test (OP).
I Animal groups
Experiments were performed on white Wistar male rats weighing 180–200 g between 10 and 16 hours, the control and experimental groups consisted of 10 animals. Rats were kept in the vivarium with free access to food and water.
II Drugs and drug administration
All investigated substances are received from firm "Sigma". Distilled water (control) and test substances were injected intramuscularly (i.m.) in a volume of 0.2-0.3 ml once for 30 minutes before testing. Anesthesia of the gastric mucosa was caused by intragastric injection of lidocaine (1 ml of 1%) or hexamethonium (0.2 mg/kg) [5, 6].
III Porsolt test
Behavioral depression in rats was studied in the standard two-day swimming test of Porsolt [7, 8]. For examination in swimming test, the rat is placed in a glass cylinder with water (at a water temperature of 22°C). On the first day, during 15 minutes of forced swimming, the immobilization time of rats is not fixed. On the second day of the experiment, the total time of immobilization of rats is determined during 5 minutes of re-swimming (all immobilization periods are fixed, when the rat become immobile or makes only small movements with the paws to maintain equilibrium). Distilled water (control) and test substances were injected intramuscularly (i.m.) in a volume of 0.2-0.3 ml once for 30 minutes before testing. The antidepressant activity of amitriptyline and phenylephrine, as well as combinations of amitriptyline with phenylephrine at threshold dose of 0.02 mg/kg, was assessed by decreasing the total immobilization time over 5 minutes of re-swimming compared to the control.
The standard maximum antidepressant effect (three-fold decrease in the immobilization time in the Porsolt test) was induced by intramuscular injection of amitriptyline at the lowest effective dose of 30 mg/kg administered 3 times a day (after 1 hour, 5 hours and 23 hours after the first swimming) [7]. For the purpose of quantitative assessment of the the maximum antidepressant activity of amitriptyline at its single administration in combination with phenylephrine in a threshold dose of 0.02 mg/kg, the minimum effective dose of amitriptyline was determined, which reduced the immobilization time in the Porsolt test 3-fold.
IV Pentylenetetrazole-induced seizures test
Pentylenetetrazole-induced seizures in rats were induced by intramuscular (i.m.) administration of a toxic dose of pentylenetetrazole (PTZ) (70 mg/kg) [10]. Estimation of severity of PTZ-induced seizures was carried out according to the generally accepted 6-point scale of Racine [11]. Stage 0 - no reaction; stage 1 - facial automatisms, twitching of ears, twitching of individual muscles, stage 2 - clonus of fore or hind paws, stage 3 - clonus of hind paws with rising, loss of posture, stage 4 - strong clonus of fore and hind paws, jumps with falling to the side, stage 5 - clonic-tonic convulsions, stage 6 - tonic convulsions. In case of recurrence of seizures, their severity increases by 0.5 points. Seizures are recorded within 30 minutes after the administration of PTZ.
Phenylephrine and diazepam, as well as the combinations of diazepam in a dose of 3 mg/kg and 10 mg/kg with phenylephrine in a dose of 0.3 mg/kg, were orally administered in 1.0 ml volume with a hard metal probe, 45 minutes before the introduction of PTZ. The animals of the control group were orally administered 1 ml of distilled water. For diazepam, phenylephrine, a combination of diazepam with phenylephrine, and control, the mean severity of seizures in points was determined, as well as the number of rats with generalized seizures (4-6 points) and local clonic seizures (2-3 points) as % of the total number of rats in a group. The anticonvulsant effect of substances was evaluated by decreasing the mean severity of PTZ-induced seizures, and by decreasing the number of rats with generalized clonic-tonic and local clonic PTZ-induced seizures compared with the control.
V Open-field test
The sedative effect of the substances was investigated in open field test (OP) [4, 9]. In the OP test, the locomotor activity of rats was determined. The animals are placed in the center of a square illuminated field (1x1 m) and the total walking time (horizontal activity) is recorded for 3 minutes, as well as number of standing on the hind legs (rearing) with or without support of wall (vertical activity). Registration of horizontal and vertical motor activity was carried out 40 minutes after the administration of the test substance. For the purpose of quantitative assessment of the locomotor activity for each dose of the test substance, the mean horizontal and mean vertical activity was calculated. The sedative effect of amitriptyline, diazepam, as well as combinations of amitriptyline and diazepam with phenylephrine, was assessed by decreasing the mean horizontal and vertical activity in the OP test in %, as compared with the rats of the control group.
VI Additional tests
To obtain additional data on the mechanism of elimination by phenylephrine of the side sedative effects of amitriptyline and diazepam, 30 minutes before i.m. administration of drug combination with phenylephrine, anesthesia of the gastric mucosa was performed by intragastric administration of lidocaine (1 ml 1%) or hexametonium (0.2 mg/kg) [5, 6].
VII Statistical data analysis
The data of the experiments on the model of PTZ-induced seizures were statistically processed using the Fisher test (frequency of seizures) and the Mann - Whitney U-test (severity of seizures). The data of the experiments in the OP test were statistically processed using the Student's t-test.
Results of the experiments and their discussion
I Effects on the antidepressant effect of amitriptyline
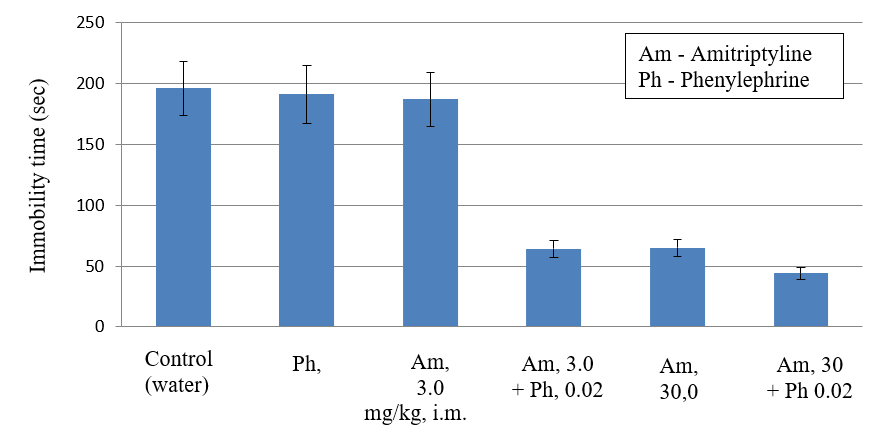
It is known that acute systemic (i.m. or i.v.) administration of amitriptyline in high doses of 10-30 mg/kg only partially eliminates behavioral depression in the standard Porsolt test, which is a model of depression in rats resistant to single administration of antidepressants [7, 8]. In our experiments, a single i.m. administration of amitriptyline to rats at high doses of 10-30 mg/kg also causes a weak antidepressant effect, since only 1.3-1.7 times less than the control reduces the immobilization time in the Porsolt test (Table 1). Amitriptyline in small doses of 0.3-3 mg/kg, amounting to 1/10-1/100 of the maximum dose of amitriptyline, practically does not change the immobilization time in the Porsolt test. As can be seen from (Table 1), the maximum antidepressant effect (a three-fold decrease in the immobilization time in the Porsolt test, p <0.05) was caused only by subacute administration of amitriptyline in the lowest effective dose of 30 mg/kg, administered 3 times a day (after 1 hour, 5 hours and 23 hours after the first swimming), as well as in the classic Porsolt experiment [7].
Phenylephrine with a single intramuscular (i.m.) administration in doses of 0.06 mg/kg and 0.2 mg/kg causes a slight antidepressant effect, as only 1.3 to 1.6 times less than the control reduces the immobilization time in the Porsolt test. Phenylephrine in a threshold dose of 0.02 mg/kg, 1/10 of its maximum dose, is completely devoid of antidepressant activity, since it does not change the immobilization time in the Porsolt test (Table 1). The combined intramuscular administration of amitriptyline in low doses of 0.3 and 1 mg/kg with phenylephrine in a threshold dose of 0.02 mg/kg causes a weak antidepressant effect (1.3-1.7 times less than the immobilization time in the Porsolt test, Table 1), analogous to the antidepressant effect when administered alone amitriptyline and phenylephrine in the maximum doses. An increase in the dose of amitriptyline up to 3 mg/kg (minimum effective dose) in combination with phenylephrine (0.02 mg/kg) leads to the development of the maximum antidepressant effect, since similar to the subacute administration of a standard (30 mg/kg) minimum effective dose of amitriptyline, in 3 times reduces the immobilization time in the Porsolt test (p <0.05, Table 1). The combined intramuscular injection of amitriptyline in a high dose of 30 mg/kg with phenylephrine in a threshold dose of 0.02 mg/kg causes the greatest antidepressant effect, which exceeds by 1.5 times the maximum antidepressant effect caused by the subacute administration of a standard effective dose of amitriptyline (30 mg/kg) (in 4.6 times reduces the immobilization time in the Porsolt test (p <0.01, table 1).
Table 1: Antidepressant effect of amitriptyline, phenylephrine and their combinations in the Porsolt test.
|
Substance, mg/kg* |
The duration of immobilization in the Porsolt test, sec |
|
Control (distilled water), i.m. |
196±22 |
|
Amitriptyline, 0.3, i.m. Amitriptyline, 1.0, i.m. Amitriptyline, 3.0, i.m. Amitriptyline, 10, i.m. Amitriptyline, 30, i.m. Amitriptyline, 30, i.m.** |
193±20 190±23 187±22 155±18 115±14 65±71 |
|
Phenylephrine, 0.02, i.m Phenylephrine, 0.06, i.m. Phenylephrine, 0.2, i.m. |
191±24 157±19 120±15 |
|
Amitriptyline, 0.3, i.m. + phenylephrine, 0.02, i.m. |
145±16 |
|
Amitriptyline, 1.0, i.m. + phenylephrine, 0.02, i.m. |
111±14 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. |
64±71 |
|
Amitriptyline, 30.0 i.m. + phenylephrine, 0.02, i.m. |
44±52 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. + lidocaine, 1 ml 1%, i.g. |
175±19 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
171±20 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. + lidocaine, 1% 1 ml 1%, i.g. |
162±25 |
|
Amitriptyline, 30,0 i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
152±23 |
*Introduced once for 30 minutes before re-swimming in the Porsolt test.
**Introduced subacute, three times a day (after 1 hour, 5 hours and 23 hours after the first swimming in the Porsolt test).
1p <0.05 compared with the control; 2p <0.01 compared with the control (Student's t-test).
Figure 1: Antidepressant effect of amitriptyline, phenylephrine and their combinations in the Porsolt test (in rats). The anti-depression effect of drugs on the durations of immobility in the forced swim test. During the 5 min test, the duration of immobility was observed and measured.
Table 2: Effect of amitriptyline, phenylephrine and their combinations on the behavior of rats in the open field test.
|
Substance, mg/kg |
Horizontal activity* |
Vertical activity** |
|
Control (distilled water), i.m. |
24.3±2.7 |
8.1±1.5 |
|
Amitriptyline, 3.0, i.m. Amitriptyline, 10, i.m. Amitriptyline, 30, i.m. |
21.0±2.7 7.1.±0.91 4.0.±0.61 |
8.4±1.8 4.0±0.51 3.2±0.41 |
|
Phenylephrine, 0.02, i.m. |
29.±3.5 |
9.5±1.9 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. |
26±2.9 |
8.7± 1.6 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. |
20±2.5 |
7.4± 1.6 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02 i.m. + lidocaine, 1 ml 1%, i.g. |
4.3.±0.61 |
3.4±0.51 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
4.7±0.71 |
3.6±0.61 |
*Total walking time (in seconds).
**Number of stands-up on the hind legs.
1p <0.05 in comparison with the control (Student's t-test).
Thus, a single combined intramuscular injection of amitriptyline in a submaximal dose of 3 mg/kg, as well as a high dose of 30 mg/kg with phenylephine in a threshold, alone ineffective dose of 0.02 mg/kg, allows to obtain the maximum antidepressant effect, impossible with a single application amitriptyline in a high dose of 30 mg/kg. It is important to note that the combined use of amitriptyline with phenylephrine can significantly accelerate the onset of the maximum antidepressant effect of amitriptyline in the Porsolt test, resistant to a single injection of antidepressants.
Preliminary anesthesia of the gastric mucosa with lidocaine, as well as the blockade of its intramural ganglions with hexamethonium, which suppress the stimulation of the afferents of the gastric mucosa with adrenaline and phenylephrine, completely eliminated the maximum antidepressant effect of combinations of amitriptyline with phenylephrine (Table 1) [5, 6]. Consequently, phenylephrine in the threshold dose as a result of stimulation of the afferents of the gastric mucosa potentiates weak antidepressant effects with acute administration of amitriptyline to the maximum level achieved only with subacute administration of amitriptyline in a high dose of 30 mg/kg.
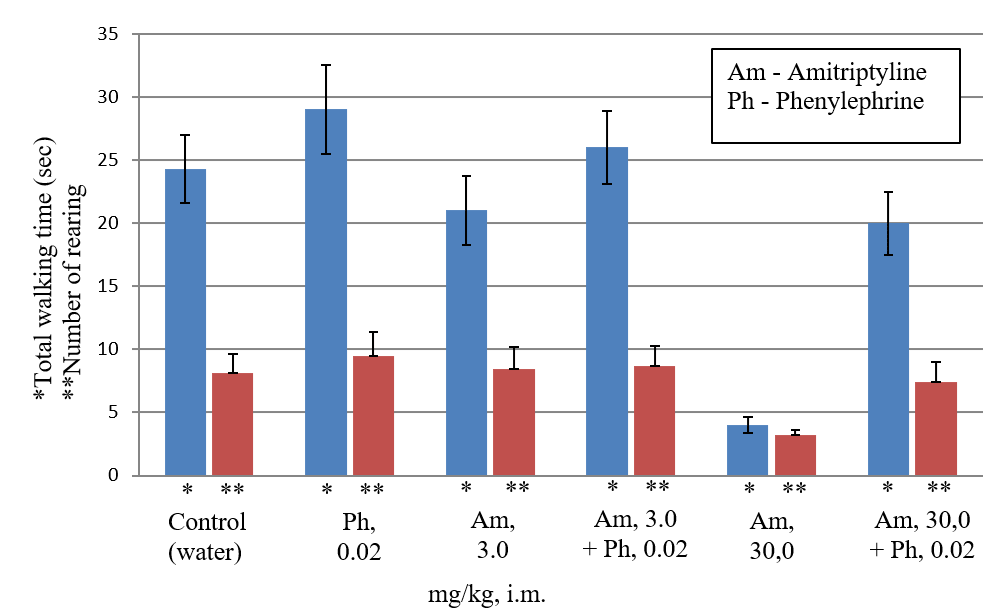
Figure 2: Estimation of the amitriptyline side effect (sedation) in the "open field" test. The total walking time (horizontal activity) is recorded for 3 minutes, as well as number of standing on the hind legs (rearing) with or without support of wall (vertical activity). The sedative effect of amitriptyline, as well as combinations of amitriptyline with phenylephrine, was assessed by decreasing the mean horizontal and vertical activity in the "open field" test in %, as compared with the rats of the control group.
Previously, we showed that antidepressant and analgesic effects caused by the introduction of high doses of epinephrine and phenylephrine are explained by the stimulation of sub-diaphragmatic gastric vagal afferents with adrenomimetics [5, 6]. Therefore, it can be assumed that weak central antidepressant effects caused by acute administration of amitriptyline are potentiated by stimulation of sub-diaphragmatic gastric vagal afferents with threshold doses of phenylephrine.
It is known that a single or subacute introduction of a high dose of amitriptyline causes side sedative effect in rats, since it significantly reduces motor activity in the open field test [9, 12, 13]. As can be seen from (Table 2), i.m. administration of amitriptyline in high doses of 10 and 30 mg/kg 3-6 times (p <0.05) decreases horizontal activity in comparison with the control, and also reduces the vertical activity 2 - 2.5 times in the open field test in rats. Therefore, the results of our experiments confirm the significant sedative effect of high doses of amitriptyline in the open field test. Amitriptyline in a submaximal dose of 3 mg/kg practically does not change the motor activity in the open field test (Table 2). Combined i.m. administration of amitriptyline in submaximal dose of 3 mg/kg and high dose of 30 mg/kg with phenylephrine in the threshold dose (0.02 mg/kg) does not have a sedative effect, since it does not reduce motor activity in the open field test compared with the control (Table 2). Consequently, phenylephrine causes the potentiation of only antidepressant, but not side sedative effect of the submaximal dose of amitriptyline. Moreover, phenylephrine completely eliminates the side sedation effect of a high dose of amitriptyline.
Preliminary anesthesia of the gastric mucosa with lidocaine, as well as the blockade of its intramural ganglions with hexamethonium, completely restored the sedative effect of a high dose of amitriptyline, despite its combined administration with phenylephrine (Table 2). Consequently, phenylephrine in the threshold dose eliminates the sedative effect of amitriptyline in a high dose as a result of stimulation of the afferents of the gastric mucosa. It follows that stimulation of the vagal afferents of the gastric mucosa by threshold doses of phenylephrine first leads to potentiation of the antidepressant effect of amitriptyline in a submaximal dose of 3 mg/kg without the development of a sedative effect and, secondly, potentiates the antidepressant effect and eliminates the sedative effect of amitriptyline in high dose of 30 mg/kg.
It is known that amitriptyline and other tricyclic antidepressants in high doses, along with sedative action, also have anxiogenic and stressogenic effects that can lead to exacerbation of depression in the initial period of antidepressant use and, as a consequence, the abolition of antidepressants [14, 15]. Combined use of phenylephrine in a threshold dose with amitriptyline and other antidepressants in a submaximal dose (1/3-1/10 of the maximum therapeutic dose) allows to obtain a fast and safe antidepressant effect, impossible with antidepressants in themselves in a high dose that causes side sedative, stressogenic and anxiogenic action. We believe that combined use of phenylephrine in a threshold dose with amitriptyline and other antidepressants at high therapeutic doses will lead to the elimination of sedative as well as anxiogenic and stressogenic action of antidepressants and will accelerate the onset of their antidepressant effect, which is especially important in the initial, most dangerous period of their use in patients with depression.
II Effects on the anticonvulsant effect of diazepam
It is known that diazepam after the systemic intraperitoneal (i.p.) and intramuscular (i.m.) administration in high doses of 5-10 mg/kg completely eliminates generalized clonic-tonic pentylenetetrazole-induced seizures in rats, but prevents local clonic pentylenetetrazole-induced seizures in only a small part rats [4, 16, 17]. In the present experiment, i.m. administration of pentylenetetrazole (PTZ) in a toxic dose of 70 mg/kg in 6-8 min causes generalized clonic-tonic seizures with a severity of 4-6 points in 100% of the rats of the control group (intravenous injection of 1 ml of distilled water). The average severity of seizures in the rats of the control group was 4.9 ± 0.5 points (Table 3). The rats of the control group 5 minutes before the diazepam administration were highly active in the "open field" test (mean horizontal activity 15.0 ± 1.9 sec, and the average vertical activity 5.0 ± 0.6 (number of stands-up on the hind legs).
Table 3: Effect of diazepam, phenylephrine and their combinations on severity of pentylenetetrazole-induced seizures, as well as the number of rats with clonic-tonic and clonic seizures.
|
Substance |
Dose, i.g. mg/kg |
Average severity of PTZ-induced seizures, in points |
The number of rats with PTZ-induced seizures in % of the total number of rats in the group |
|
|
Clonico-tonic seizures* |
Clonic seizures** |
|||
|
Control (distilled water) |
|
4.9±0.5 |
100% |
100% |
|
Diazepam |
3 |
3.5±0.5 |
50% |
100% |
|
Diazepam |
10 |
2.4±0.42 |
0%1 |
86% |
|
Phenylephrine |
0.3 |
3.5±0.5 |
60% |
100% |
|
1.0 |
2.5±0.52 |
14%2 |
100% |
|
|
Diazepam + phenylephrine |
3 0.3 |
1.5±0.31 |
0%1 |
28%1 |
|
Diazepam + phenylephrine |
10 0.3 |
0.9±0.21 |
0%1 |
0%1 |
|
Diazepam + phenylephrine + lidocaine, 1 ml 1% |
10 0.3 |
2.0±0.32 |
0%1 |
73% |
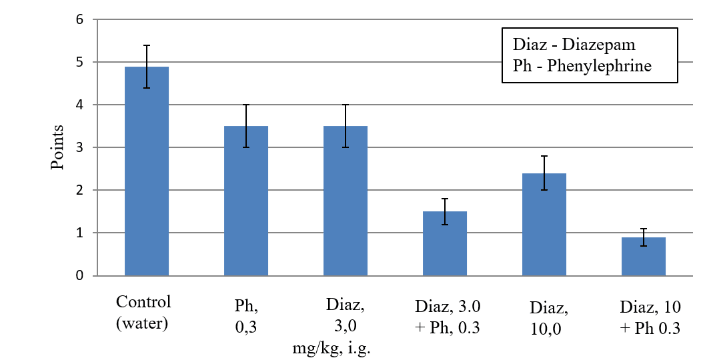
As can be seen from (Table. 3), diazepam in a submaximal dose of 3 mg/kg with oral administration causes a weak and unreliable anticonvulsant effect, since it eliminates clonic-tonic convulsions in only 50% of rats and reduces the average severity of coronary convulsions by 28% (Table 3). Diazepam, when administered orally in a high dose of 10 mg/kg, significantly (p <0.05) by 51% compared with the control reduces the average severity of PTZ-induced seizures. In this dose, diazepam most effectively inhibits the development of generalized clonic-tonic seizures with a severity of 4-5 points in 100% of rats (p <0.01), but it is unreliable in only 14% of rats to prevent the development of local clonic seizures with a severity of 2-3 points (Table 3). It follows that diazepam in a high dose of 10 mg/kg with oral administration, as well as in systemic administration, suppresses most effectively the generalized clonic-tonic PTZ-induced seizures, but only a small proportion of rats prevent the development of local clonal PTZ-induced seizures resistant to action diazepam and other antiepileptic agents [4, 16, 17].
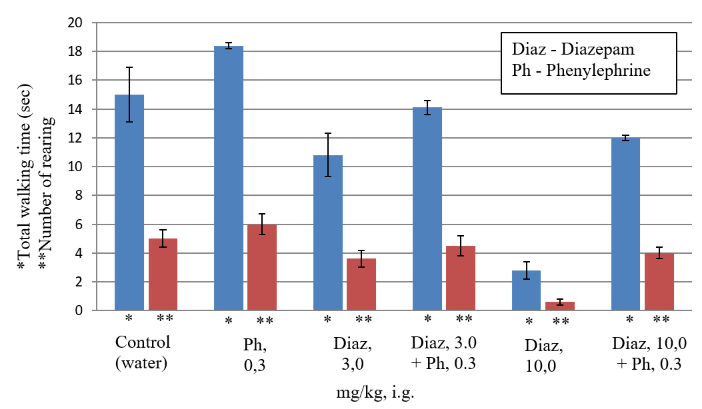
It is known that the anticonvulsant effect of diazepam develops only in high doses, causing side sedative effect, which manifests itself in a significant decrease in horizontal and vertical motor activity in the "open field" test (OP test) [4, 18, 19]. As can be seen from the (Table 4), oral administration of diazepam in a submaximal dose of 3 mg/kg slightly changes the locomotor activity in the OP test. Diazepam in a high dose of 10 mg/kg significantly (p <0.05–0.01) compared with the control, respectively, 5 and 9 times reduces the horizontal and vertical locomotor activity in the OP test. Consequently, the results of our experiments confirm the presence of a strong sedative effect after oral administration of high doses of diazepam in the OP test.
Phenylephrine in an average dose of 0.3 mg/kg after oral administration causes a weak and unreliable anticonvulsant effect, since it eliminates clonic-tonic seizures in only 40% of rats and reduces the average severity of PTZ-induced seizures by 28% (Table 3). Phenylephrine in a high dose of 1 mg/kg, like diazepam, causes a reliable anticonvulsant effect, since it reduces the average severity of PTZ-induced seizures by 49%, and also eliminates clonic-tonic seizures in 86% of rats (p <0.05, Table 3). In this dose, phenylephrine does not decrease the number of rats with clonal PTZ-induced seizures (Table 3). In contrast to diazepam, phenylephrine in average dose of 0.3 mg/kg and a high dose of 1.0 mg/kg is devoid of side sedation, as it does not reliably alter the motor activity in the OP test in rats (Table 4).
Figure 3: Anticonvulsant effect of diazepam, phenylephrine, and their combinations in the pentylenetetrazole (PTZ)-induced seizures (in rats, 70 mg/kg, i.m.). Seizures are recorded within 30 minutes after the administration of PTZ. Estimation of severity of PTZ-induced seizures was carried out according to the generally accepted 6-point scale of Racine.
Table 4: Effect of diazepam, phenylephrine and their combinations on the locomotor activity of rats in the open field test.
|
Substance |
Dose, i.g. mg/kg |
Horizontal activity* |
Vertical activity** |
|
Control (distilled water) |
|
15.0±1.9 |
5.0±0.6 |
|
Diazepam |
3 |
10.8±1.5 |
3.6±0.6 |
|
Diazepam |
10 |
2.8±0.62 |
0.6±0.21 |
|
phenylephrine |
0.3 |
18.4±0.2 |
6.0±0.7 |
|
1.0 |
20.5±0.3 |
6.6±0.8 |
|
|
Diazepam + phenylephrine |
3 0.3 |
14.1±0.5 |
4.5±0.7 |
|
Diazepam + phenylephrine |
10 0.3 |
12.0±0.2 |
4.0±0.6 |
|
Diazepam + phenylephrine + lidocaine, 1 ml 1% |
10 0.3 |
3.0±0.62 |
0.6±0.21 |
*Total walking time (in seconds.
**Number of stands-up on the hind legs.
1p <0.01 compared with the control.
2
As can be seen from (Table 3), the combined oral administration of diazepam in submaximal dose of 3 mg/kg with phenylephrine in average alone ineffective dose 0.3 mg/kg reliably increases anticonvulsant activity of diazepam 2.3 times (mean severity of convulsions decreases from 3.5 ± 0.5 to 1.5 ± 0.3 points, Table 3). It is important to note that this combination of diazepam with phenylephrine causes the maximum anticonvulsant effect, impossible with the use of diazepam by themselves even in a high dose of 10 mg/kg, since it eliminates not only generalized clonic-tonic seizures in 100% of rats but also local clonic seizures in 72% of rats (p <0.01, Table 3). The combined oral administration of diazepam in submaximal dose of 3 mg/kg with phenylephrine in average dose of 0.3 mg/kg does not cause sedation, since it does not reliably reduce the motor activity of rats in the OP test compared to the control (Table 4).
Figure 4: Estimation of the diazepam side effect (sedation) in the "open field" test. The total walking time (horizontal activity) is recorded for 3 minutes, as well as number of standing on the hind legs (rearing) with or without support of wall (vertical activity). The sedative effect of diazepam, as well as combinations of diazepam with phenylephrine, was assessed by decreasing the mean horizontal and vertical activity in the OP test in %, as compared with the rats of the control group
The combined oral administration of diazepam in high dose of 10 mg/kg with phenylephrine in average alone ineffective dose 0.3 mg/kg reliably increases anticonvulsant activity of diazepam 2.6 times (the average severity of convulsions decreases from 2.4 ± 0.4 to 0.9 ± 0.2 points, Table 3) This combination of diazepam with phenylephrine causes the greatest possible anticonvulsant effect, since it eliminates not only generalized clonic-tonic seizures in 100% of rats, but also local clonic seizures in 100% of rats (p <0.01, Table 3). The combined oral administration of diazepam in high dose of 10 mg/kg with phenylephrine in average dose of 0.3 mg/kg almost completely eliminates the sedative effect of diazepam, since it does not reliably reduce the motor activity of rats in the OP test compared to the control (Table 4).
Preliminary anesthesia of the gastric mucosa by 1% lidocaine, which suppresses the stimulation of the afferents of the gastric mucosa with epinephrine and phenylephrine, completely eliminates the potentiation of anticonvulsant effects of diazepam in combination with phenylephrine, and also completely restores the sedative effect of diazepam, despite its combined administration with phenylephrine (Tables 3, 4) [6]. Consequently, stimulation of the afferents of the gastric mucosa with phenylephrine not only potentiates the anticonvulsant effect of diazepam to the highest possible level, but also helps to eliminate the sedative effect of diazepam in a high therapeutic dose.
Earlier, we showed that stimulation of gastric vagal afferents with adrenaline in the threshold dose potentiates the anticonvulsant effect of diazepam in small and medium doses in the case of combined i.m. administration of these two agents without enhancing the sedative activity of diazepam [4]. Therefore, it can be assumed that in this experiment the stimulation of the vagal afferents of the stomach by phenylephrine potentiates the anticonvulsant effect of diazepam in submaximal dose (1/3 of the maximum dose, ineffective if used alone) to the maximum level impossible in the case of use of diazepam by themselves in high dose without enhancing of the sedative activity of diazepam.
Stimulation of vagal afferents of the stomach with phenylephrine causes not only the potentiation of anticonvulsant action, but also eliminates the side sedative effect of diazepam in the maximum dose. It can be assumed that the combined use of phenylephrine with diazepam and other antiepileptic drugs in submaximal and maximally high therapeutic doses is a new effective and safe way of treating temporal frontal epilepsy resistant to the action of antiepileptics.
III The mechanism of the potentiating action of phenylephrine on the effects of amitriptyline and diazepam.
It is known that systemic amitriptyline inhibits predominantly the norepinephrine reuptake and increases extracellular norepinephrine to nearly 19 times [20, 21]. Antidepressant and analgesic effects of amitriptyline and other tricyclic antidepressants are mainly associated with activation of presynaptic inhibitory brain 2-adrenergic receptors [22-26]. Diazepam has analgesic, sedative, anticonvulsant and anxiolytic effects as a result of activation of inhibitory presynaptic GABAB and postsynaptic GABAA receptors in the brain [27-29]. It is also known that in therapeutic doses tricyclic antidepressants and diazepam have a central anti-stress sympatholytic effect as a result of inhibition of the activity of RVLM and Locus ceruleus neurons, which is associated with stimulation of, respectively, inhibitory presynaptic α2 adrenergic receptors, as well as presynaptic GABAB and postsynaptic GABAA receptors of these structures [21, 25, 30-39].
Tricyclic antidepressants and diazepam in therapeutic doses cause antidepressant, analgesic, anxiolytic and anticonvulsant effects as a result of a direct decrease in the activity of neurons RVLM, Locus ceruleus, motoneurons and afferent neurons of the spinal cord, as well as neurons of the hippocampal pyramids associated with activation of inhibitory presynaptic 2-adrenergic receptors, presynaptic GABAA and postsynaptic GABAB receptors in these structures [24, 40-49]. We believe that the above-mentioned effects of therapeutic doses of amitriptyline and diazepam are a manifestation not only of their own therapeutic effect, but also the result of their central stress-protective action associated with the reinforcement of reflex inhibitory vagal GABAergic influences on RVLM, Locus ceruleus, motoneurons and spinal cord afferents , as well as neurons of the pyramids of the hippocampus. Apparently, the enhancement of reflex GABAergic inhibition can be explained by additional activation of the central link of stress-protective reflexes as a result of stimulation of presynaptic 2-adrenergic receptors under the action of amitriptyline, as well as GABAA and GABAB receptors under the action of diazepam in these brain structures.
The maximum potentiation of analgesic, antidepressant and anticonvulsant effects of compositions containing therapeutic doses of amitriptyline and diazepam with phenylephrine at the threshold dose is probably due to the increased central reflex GABAergic inhibition of neurons RVLM, Locus ceruleus, neurons of the hippocampal pyramids and spinal cord caused, on the one hand, by the peripheral stimulation of subdiaphragmal vagal afferents by phenylephrine, and on the other hand, direct stimulation of central GABA receptors by diazepam or 2-adrenergic receptors by amitriptyline.
An additional reason for the development of maximal effects in experiments on resistant models of pain, of epilepsy and depression may be a summation of central stress-protective effect of these compositions with their own submaximal therapeutic central effects of amitriptyline and diazepam, as submaximal analgesic, anti-stress, antidepressant and anticonvulsant effects of amitriptyline and diazepam associated with development of GABAergic inhibition of the same brain structures as in the case of activation of the central stress-protective reflexes. Elimination of the side sedative effect of amitriptyline and diazepam in compositions with phenylephrine can be explained not only by the elimination of anxiety, sedation and hypokinesia as components of the stress reaction to the drug, but also by direct activation of the vagus effect on the mesolimbic brain system [50, 51]. We believe that the most important element of vagal potentiation of therapeutic effects and elimination of side effects of amitriptyline and diazepam is the suppression of drug stress caused by amitriptyline and diazepam, especially in the initial period of their use.
We have previously shown that intramuscular administration of central analgesics of fentanyl and analgin, as well as mediators of pain of cholecystokinin, glutamate, ATP, epinephrine and adenosine, weakly penetrating into the CNS, causes the maximum analgesic effect in the tail-flick test in rats [60]. Minimally effective doses of analgin and fentanyl decrease by 50-220 times with combined intramuscular administration of each of the analgesics with cholecystokinin, glutamate, ATP, adrenaline and adenosine in threshold doses, ineffective if used alone. Intragastric administration of lidocaine, as well as subdiaphragmatic gastric vagotomy completely eliminate the analgesic effects of the above combinations. It was concluded that peripherally acting mediators of pain and analgesia after systemic administration potentiate the central analgesic effect of analgin and fentanyl as a result of stimulation of chemoreceptors of vagal afferents of the gastric mucosa [3]. Therefore, we believe that not only adrenomimetics but also other peripheral agonists of chemoreceptors can be used as stimulators of vagal afferents: serotonin, adenosine, ATP, acetylcholine, glutamate, CCK, ghrelin, leptin, endocannabinoids, capsaicin, and others.
Conclusion
The results of the conducted experiments showed that the peripherally acting adrenomimetic phenylephrine in threshold doses (ineffective if used alone) selectively stimulating gastric vagal afferents, in case combined oral administration, potentiates the anticonvulsant effect of diazepam and the antidepressant effect of amitriptyline in submaximal and high therapeutic doses to the maximum level impossible in their application by themselves, and eliminates their side sedative effect in high doses. Since the preliminary anesthesia of the gastric mucosa by 1% lidocaine completely eliminates the effect of phenylephrine on the therapeutic and side effects of diazepam and amitriptyline, it can be assumed that the mechanism for potentiating therapeutic effects and eliminating the side effects of high doses of the indicated CNS agents is based on the same mechanism - stimulation of afferents (probably vagus) of the gastric mucosa by phenylephrine.
Experimental evidence was obtained for the first time of the possibility of using the method of selective chemoreceptor stimulation of gastric vagal afferents in order to maximize the potentiation of therapeutic effects and eliminate the side effects of CNS agents in high therapeutic doses. There is reason to believe that chemoreceptor stimulation of the vagus by phenylephrine and other peripherally acting adrenomimetics potentiates to the highest possible level the therapeutic effect and reduces the side effects of high therapeutic doses of antiepileptics and antidepressants due to activation of central vagal stress-protective reflexes aimed at eliminating the drug stress that weakens therapeutic effects of CNS agents.
An additional reason for the development of maximum therapeutic effects may be the summation of the central stress-protective effects of these combinations with the own therapeutic central effects of the CNS agents. A new method of selective chemoreceptor stimulation of vagus is not inferior in efficiency to the method of electrical stimulation of vagus, but is simpler, cheaper and more affordable for mass application. It allows to obtain maximum and at the same time safe therapeutic effects in patients with severe, drug-resistant CNS diseases. The use of the method of chemoreceptor stimulation of the vagus to potentiate therapeutic effects and eliminate the side effects of high doses of the CNS agents has the advantage over all existing methods of combination therapy that do not eliminate, but often increase the side effects of CNS agents.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 01, Jul 2019Accepted: Mon 26, Aug 2019
Published: Sun 10, Nov 2019
Copyright
© 2023 Valery Gmiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.EJGM.2019.01.01
Author Info
Corresponding Author
Valery GmiroInstitute for Experimental Medicine, 197376, St. Petersburg, Acad. Pavlov St., 12, Russia
Figures & Tables
Table 1: Antidepressant effect of amitriptyline, phenylephrine and their combinations in the Porsolt test.
|
Substance, mg/kg* |
The duration of immobilization in the Porsolt test, sec |
|
Control (distilled water), i.m. |
196±22 |
|
Amitriptyline, 0.3, i.m. Amitriptyline, 1.0, i.m. Amitriptyline, 3.0, i.m. Amitriptyline, 10, i.m. Amitriptyline, 30, i.m. Amitriptyline, 30, i.m.** |
193±20 190±23 187±22 155±18 115±14 65±71 |
|
Phenylephrine, 0.02, i.m Phenylephrine, 0.06, i.m. Phenylephrine, 0.2, i.m. |
191±24 157±19 120±15 |
|
Amitriptyline, 0.3, i.m. + phenylephrine, 0.02, i.m. |
145±16 |
|
Amitriptyline, 1.0, i.m. + phenylephrine, 0.02, i.m. |
111±14 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. |
64±71 |
|
Amitriptyline, 30.0 i.m. + phenylephrine, 0.02, i.m. |
44±52 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. + lidocaine, 1 ml 1%, i.g. |
175±19 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
171±20 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. + lidocaine, 1% 1 ml 1%, i.g. |
162±25 |
|
Amitriptyline, 30,0 i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
152±23 |
*Introduced once for 30 minutes before re-swimming in the Porsolt test.
**Introduced subacute, three times a day (after 1 hour, 5 hours and 23 hours after the first swimming in the Porsolt test).
1p <0.05 compared with the control; 2p <0.01 compared with the control (Student's t-test).
Table 2: Effect of amitriptyline, phenylephrine and their combinations on the behavior of rats in the open field test.
|
Substance, mg/kg |
Horizontal activity* |
Vertical activity** |
|
Control (distilled water), i.m. |
24.3±2.7 |
8.1±1.5 |
|
Amitriptyline, 3.0, i.m. Amitriptyline, 10, i.m. Amitriptyline, 30, i.m. |
21.0±2.7 7.1.±0.91 4.0.±0.61 |
8.4±1.8 4.0±0.51 3.2±0.41 |
|
Phenylephrine, 0.02, i.m. |
29.±3.5 |
9.5±1.9 |
|
Amitriptyline, 3.0, i.m. + phenylephrine, 0.02, i.m. |
26±2.9 |
8.7± 1.6 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. |
20±2.5 |
7.4± 1.6 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02 i.m. + lidocaine, 1 ml 1%, i.g. |
4.3.±0.61 |
3.4±0.51 |
|
Amitriptyline, 30, i.m. + phenylephrine, 0.02, i.m. + hexamethonium, 0.2, i.g. |
4.7±0.71 |
3.6±0.61 |
*Total walking time (in seconds).
**Number of stands-up on the hind legs.
1p <0.05 in comparison with the control (Student's t-test).
Table 3: Effect of diazepam, phenylephrine and their combinations on severity of pentylenetetrazole-induced seizures, as well as the number of rats with clonic-tonic and clonic seizures.
|
Substance |
Dose, i.g. mg/kg |
Average severity of PTZ-induced seizures, in points |
The number of rats with PTZ-induced seizures in % of the total number of rats in the group |
|
|
Clonico-tonic seizures* |
Clonic seizures** |
|||
|
Control (distilled water) |
|
4.9±0.5 |
100% |
100% |
|
Diazepam |
3 |
3.5±0.5 |
50% |
100% |
|
Diazepam |
10 |
2.4±0.42 |
0%1 |
86% |
|
Phenylephrine |
0.3 |
3.5±0.5 |
60% |
100% |
|
1.0 |
2.5±0.52 |
14%2 |
100% |
|
|
Diazepam + phenylephrine |
3 0.3 |
1.5±0.31 |
0%1 |
28%1 |
|
Diazepam + phenylephrine |
10 0.3 |
0.9±0.21 |
0%1 |
0%1 |
|
Diazepam + phenylephrine + lidocaine, 1 ml 1% |
10 0.3 |
2.0±0.32 |
0%1 |
73% |
Table 4: Effect of diazepam, phenylephrine and their combinations on the locomotor activity of rats in the open field test.
|
Substance |
Dose, i.g. mg/kg |
Horizontal activity* |
Vertical activity** |
|
Control (distilled water) |
|
15.0±1.9 |
5.0±0.6 |
|
Diazepam |
3 |
10.8±1.5 |
3.6±0.6 |
|
Diazepam |
10 |
2.8±0.62 |
0.6±0.21 |
|
phenylephrine |
0.3 |
18.4±0.2 |
6.0±0.7 |
|
1.0 |
20.5±0.3 |
6.6±0.8 |
|
|
Diazepam + phenylephrine |
3 0.3 |
14.1±0.5 |
4.5±0.7 |
|
Diazepam + phenylephrine |
10 0.3 |
12.0±0.2 |
4.0±0.6 |
|
Diazepam + phenylephrine + lidocaine, 1 ml 1% |
10 0.3 |
3.0±0.62 |
0.6±0.21 |
*Total walking time (in seconds.
**Number of stands-up on the hind legs.
1p <0.01 compared with the control.
2
References
- Gmiro VE, Serdyuk SE (2007) Epinephrine potentiates antipsychotic, but not cataleptogenic effect of haloperidol in rats. Bull Exp Biol Med 143: 617-619. [Crossref]
- Serdyuk SE, Gmiro VE (2007) Epinephrine potentiates the analgesic and antidepressant effects of amitriptyline as a result of stimulation of the gastric mucosal afferents. Bull Exp Biol Med 144: 692-694. [Crossref]
- Serdyuk SE, Gmiro VE (2013) Peripherally acting mediators of pain and analgesia potentiate the central analgesic effects of fentanyl and analgin (dipyrone) in rats. Neurosci Behavi Physiol 43: 1028-1031.
- Serdyuk SE, Gmiro VE (2013) Stimulation of gastric mucosal afferents with adrenaline potentiates the anticonvulsive but not the sedative action of diazepam in rats. Neurosci Behavi Physiol 43: 963-966.
- Serdyuk SE, Gmiro VE (1995) The Participation of gastric afferents in reflex mechanisms of fast adaptation to stress exposures. Fiziol Zh Im IM Sechenova 81: 40-51. [Crossref]
- Serdyuk SE, Gmiro VE (1997) The analgesic and antidepressant action of adrenaline-induced stress in the endogenous activation of the gastric afferent systems in rats. Ross Fiziol Zh Im IM Sechenova 83 : 111-120. [Crossref]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1987) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379-391. [Crossref]
- Weinstock M, Poltyrev T, Bejar C, Youdim MB (2002) The Effect of TV3326, a novel monoamine-oxidase cholinesterase inhibitor, in rat models of anxiety and depression. Psychopharmacology (Berl) 160: 318-324. [Crossref]
- Epstein OI, Molodavkin GM, Voronina TA, Sergeeva SA (2003) Antidepressant properties of proproten and amitriptyline: a comparative experimental study. Bull Exp Biol Med 7: 123-124. [Crossref]
- Gmiro VE, Serdyuk SE (2000) A comparative study of the NMDA-block1ng activity and safety of mono- and bis-cationic compounds in experimental animals. Eksp Klin Farmakol 63: 3-8.
- Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281-294. [Crossref]
- Pietrasiewicz T, Zebrowska-Lupina I (1996) Studies on the interaction of antidepressant drugs with adrenocorticotropic hormone or prednisone in rats. Pol J Pharmacol 48: 145-152. [Crossref]
- Stein D, Peri T, Edelstein E, Elizur A, Floman Y (1996) The efficacy of amitriptyline and acetaminophen in the management of acute low back pain. Psychosomatics 37: 63-70. [Crossref]
- Hindmarch I (1998) The behavioural toxicity of antidepressants: effects on cognition and sexual function. Int Clin Psychopharmacol 13: S5-S8. [Crossref]
- Londborg PD, Smith WT, Glaudin V, Painter JR (2000) Short-term cotherapy with clonazepam and fluoxetine: anxiety, sleep disturbance and core symptoms of depression. J Affect Disord 61: 73-79. [Crossref]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM (2000) Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology 39: 1184-1196. [Crossref]
- Haugvicová R, Kubová H, Mares P (2002) Does vigabatrin possess an anticonvulsant action against pentylenetetrazol-induced seizures in developing rats? Physiol Res 51: 363-370. [Crossref]
- Hughes RN (1993) Effects on open-field behavior of diazepam and buspirone alone and in combination with chronic caffeine. Life Sci 53: 1217-1225. [Crossref]
- Keane PE, Simiand J, Morre M, Biziere K (1988) Tetrazepam: a benzodiazepine which dissociates sedation from other benzodiazepine activities. I. Psychopharmacological profile in rodents. J Pharmacol Exp Ther 245: 692-698. [Crossref]
- Engleman EA, Wong DT (1996) Regulation of extracellular concentrations of norepinephrine in hypothalamus of the conscious rat: effect of amitriptyline. Chin J Physiol 39: 9-13. [Crossref]
- Leighton HJ (1982) Quantitative assessment of the pre- and postsynaptic alpha adrenoceptor antagonist potency of amitriptyline. J Pharmacol Exp Ther 220: 299-304. [Crossref]
- Cervo L, Grignaschi G, Samanin R (1990) Alpha 2-adrenoceptor blockade prevents the effect of desipramine in the forced swimming test. Eur J Pharmacol 175: 301-307. [Crossref]
- Grandoso L, Pineda J, Ugedo L (2004) Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices. Neuropharmacology 46: 815-823. [Crossref]
- Gray AM, Pache DM, Sewell RD (1999) Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol 378: 161-168. [Crossref]
- Ozdogan UK, Lahdesmaki J, Mansikka H, Scheinin M (2004) Loss of amitriptyline analgesia in alpha 2A-adrenoceptor deficient mice. Eur J Pharmacol 485: 193-196. [Crossref]
- Schramm NL, McDonald MP, Limbird LE (2001) The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci 21: 4875-4882. [Crossref]
- Dalvi A, Rodgers RJ (2001) Anxiolytic effects of valproate and diazepam in mice are differentially sensitive to picrotoxin antagonism. Pharmacol Biochem Behav 68: 23-32. [Crossref]
- Kralic JE, O'Buckley TK, Khisti RT, Hodge CW, Homanics GE et al. (2002) GABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidem. Neuropharmacology 43: 685-694. [Crossref]
- Morimoto K, Holmes KH, Goddard GV (1987) Kindling-induced changes in EEG recorded during stimulation from the site of stimulation. III. Direct pharmacological manipulations of the kindled amygdala. Exp Neurol 97: 17-34. [Crossref]
- Agelink MW, Majewski TB, Andrich J, Mueck-Weymann M (2002) Short-term effects of intravenous benzodiazepines on autonomic neurocardiac regulation in humans: a comparison between midazolam, diazepam, and lorazepam. Crit Care Med 30: 997-1006. [Crossref]
- Amano M, Kubo T (1993) Involvement of both GABAA and GABAB receptors in tonic inhibitory control of blood pressure at the rostral ventrolateral medulla of the rat. Naunyn Schmiedebergs Arch Pharmacol 348: 146-153. [Crossref]
- Chan RK, Sawchenko PE (1998) Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371-387. [Crossref]
- Farmer MR, Vaile JC, Osman F, Ross HF, Townend JN et al. (1998) A central gamma-aminobutyric acid mechanism in cardiac vagal control in man revealed by studies with intravenous midazolam. Clin Sci (Lond) 95: 241-248. [Crossref]
- Huangfu D, Goodwin WB, Guyenet PG (1995) Sympatholytic effect of tricyclic antidepressants: site and mechanism of action in anesthetized rats. Am J Physiol 268: R1429-R1441. [Crossref]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Hildebrandt G et al. (1999) Influence of age on the parasympatholytic property of tricyclic antidepressants. Psychiatry Res 85: 199-207. [Crossref]
- Low PA, Opfer-Gehrking TL (1992) Differential effects of amitriptyline on sudomotor, cardiovagal, and adrenergic function in human subjects. Muscle Nerve 15: 1340-1344. [Crossref]
- Sazbo B, Schultheiss A (1990) Desipramine inhibits sympathetic nerve activity in the rabbit. Naunyn Schmiedebergs Arch Pharmacol 342: 469-476.
- Simson PE, Weiss JM (1989) Peripheral, but not local or intracerebroventricular, administration of benzodiazepines attenuates evoked activity of locus coeruleus neurons. Brain Res 490: 236-242. [Crossref]
- Xu NS, Guo XQ, Zhang JR (1993) Inhibitory effect of diazepam or flurazepam on pressor response induced by the stimulation of midbrain in the rabbit. Sheng Li Xue Bao 45: 447-454. [Crossref]
- Abdi S, Lee DH, Chung JM (1998) The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg 87: 360-366. [Crossref]
- Baba H, Goldstein PA, Okamoto M, Kohno T, Ataka T, et al (200) Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology 92: 485-492. [Crossref]
- Becker A, Tiedge A, Grecksch GA (1997) Diazepam - its effects on the development of pentylenetetrazol kindling, related learning impairments, and neuronal cell loss. Pharmacol Res 35: 27-32. [Crossref]
- Grant MM, Weiss JM (2001) Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry 49: 117-129. [Crossref]
- Kalman BA, Kim PJ, Cole MA, Chi MS, Spencer RL (1997) Diazepam attenuation of restraint stress-induced corticosterone levels is enhanced by prior exposure to repeated restraint. Psychoneuroendocrinology 22: 349-360. [Crossref]
- Khan GM, Smolders I, Lindekens H, Manil J, Ebinger G, et al. (1999) Effects of diazepam on extracellular brain neurotransmitters in pilocarpine-induced seizures in rats. Eur J Pharmacol 373: 153-161. [Crossref]
- Loscher W (1999) Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol 58: 31-59. [Crossref]
- McQuay HJ, Carroll D, Glynn CJ (1992) Low dose amitriptyline in the treatment of chronic pain. Anaesthesia 47: 646-652. [Crossref]
- Szabo ST, Blier P (2001) Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci 13: 2077-2087. [Crossref]
- Takano K, Student JC (1978) Effect of diazepam on the gamma motor system indicated by the responses of the muscle spindle of the triceps surae muscle of the decerebrate cat to the muscle stretch. Naunyn Schmiedebergs Arch Pharmacol 302: 91-101. [Crossref]
- Manta S, El Mansari M, Debonnel G, Blier P (2013) Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 16: 459-470. [Crossref]
- Ziomber A, Thor P, Krygowska-Wajs A, Załęcki T, Moskała M et al. (2012) Chronic impairment of the vagus nerve function leads to inhibition of dopamine but not serotonin neurons in rat brain structures. Pharmacol Rep 64: 1359-1367. [Crossref]
