Spontaneous Hemothorax in Infants: An Unusual Presentation
A B S T R A C T
We report the case of a 40-day-old boy, who presented to hospital with tachypnea and sudden pallor. A pleural effusion in the left chest was observed on the chest X-ray. He was ventilated and thoracic drainage was performed because of degradation of his respiratory status. Computed Tomography as well as Magnetic Resonance Imaging supported strongly the diagnosis of mediastinal neuroblastoma. The extension assessment was negative. We opted for a conservative treatment by chemotherapy, and the courses proceeded favourably. After neoadjuvant chemotherapy, the residual mass was resected completely through a left thoracotomy. Histopathological examination established the final diagnosis of ganglioneuroblastoma. Thus, it is possible to avoid invasive treatment for massive hemothorax caused by neuroblastoma by initiating chemotherapy.
Keywords
Neuroblastoma, hemothorax, pleural effusion, chemotherapy, thoracic drainage
Background
Neuroblastoma is the most common solid tumor in newborn and the most common extracranial solid tumor in infancy. It accounts for 7-10% of all pediatric cancers. Neuroblastoma is diagnosed before one year in 40% of cases, between 1 and 2 years in 35% of cases, and after 2 years in 25% of cases [1]. Pleural effusion caused by thoracic neuroblastoma is uncommon in newborn and infants [2]. Its management depends on the location of tumor, the extent of metastatic disease and the respiratory distress at diagnosis. The authors report this clinical case to highlight the efficacy of conservative treatment in mediastinal neuroblastoma complicated by hemothorax.
Case Report
Herein we report the case of a 40-day-old infant, who presented to the pediatric emergency department with the complaints of respiratory distress of recent onset associated to a sudden pallor. The patient was a male infant at 36 weeks’ gestation with a birth weight of 3.780 kg. The pregnancy was well monitored, complicated by gestational diabetes treated by Insulin. He was born via caesarean section with good adaptation to extra-uterine life. He was hospitalized at day 7 of life for physiologic jaundice treated by phototherapy.
At admission, he was irritable, crying without signs of sepsis or dehydration. On physical inspection, anthropometry was within normal limits (weight gain of 25 grams/day). He was found to be afebrile and pale. Physical examination revealed signs of respiratory distress, including tachypnea (65 cycles/minute of respiratory rate), mild nasal flaring and intercostal recession. Dullness to percussion with decreased breath sounds on the left lung field was also noted without crackles nor wheeze. The Oxyhaemoglobin saturation was normal (98%) on room air. He had normal heart sounds and symmetrical femoral pulses. The pulse rate was 168bpm and the blood pressure was normal for age. Abdominal exploration was normal. The rest of examination was otherwise without abnormalities. The routine haematology and biochemistry parameters were within normal limits except for the decreased value of haemoglobin (4,7g/dl) and the increase value of LDH (602 IU/L). Furthermore, the C reactive protein was 10.32 mg /l, the visceral balance was correct and there was not a syndrome of inappropriate antidiuretic hormone secretion. Chest X-ray noted left sided pleural effusion with mild shift of mediastinum to the right (Figure 1).
Figure 1: Chest X-ray: Left sided pleural effusion with shift of mediastinum (arrow).
Figure 2: Chest X-ray after removal of the tube: complete clearing of the left chest.
As the respiratory condition worsened, with increased signs of distress and desaturation, the infant was transferred to the intensive care department. He was therefore ventilated, and an inter-costal drain tube was placed in the left hemithorax. On needle thoracocentesis, 140 ml of red serosanguinous fluid was aspirated. There were few lymphocytes and no atypical or malignant cells. He received a filling with physiological serum and a transfusion of phenotyped blood (20 ml/kg). The post-transfusion haemoglobin was 10 g/dl. This procedure improved the patient's respiratory status and he was extubated 24 hours after. The infant initially received antibiotic therapy which was subsequently stopped due to the negativity of the bacteriological sample. Post-procedure evaluation with chest X-ray showed a significantly increased aeration of the left lung (Figure 2).
As part of the etiological assessment of the hemothorax, a thoracic computerized tomography (CT) was performed. It revealed a voluminous tissue mass in the left posterior infra-mediastinal space extended to the subdiaphragmatic space, suggesting first and foremost a thoracic neuroblastoma with intra-channel extension and the presence of multiple imaging-defined risk factors (IDRF) (Figure 3). The upper abdominal organs were normal.
Figure 3: A) Axial, B) coronal and C) sagittal CT section showing a heteregenous solid mass involving the left posterior mediastinal space with calcifications (arrow), atelectasis of the left lower lobe and moderate pleural effusion. There is an intraspinal extension on 35mm of height of more than 50% of medullary canal (T10-T12) and widening of the posterior intercostals spaces. It pushes the aorta to the right, crosses the midline, and encases the abdominal aorta and its branches. The superior cave vein is detached anteriorly and to the right.
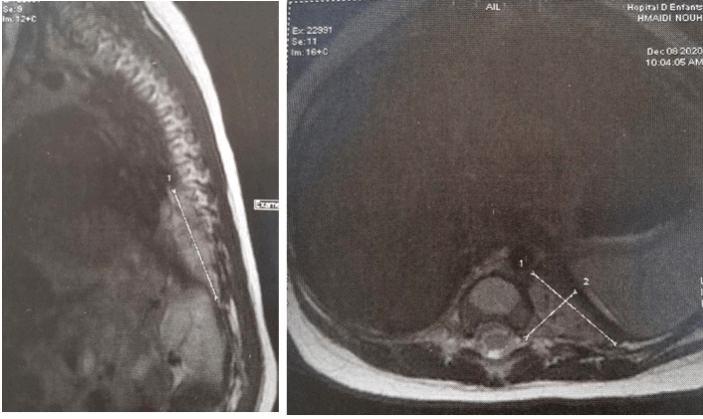
The infant was transferred secondarily to the pediatric oncology unit. A germ cell tumor was evoked because of the median location of the tumor but eliminated by normal alpha-fetoprotein (667 ng/ml) and Human Chorionic Gonadotropin (0.1mIU/ml) levels. As part of the extension workup, a Magnetic Resonance Imaging (MRI) of the spinal cord revealed a large left tissular mass involving the posterior inframediastinal space with a large intracanal extension from T10 to T12 displacing the spinal cord to the right without epiduritis (Figure 4).
Figure 4: A) MRI axial section and B) MRI sagittal section showing a hyperintense T2 left paravertebral mass with extension through the neuroforamen into the spinal canal that displaces the spinal cord to the right (arrow).
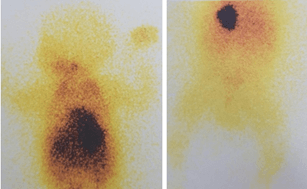
Figure 5: 131I MIBG scan shows increased radiotracer uptake in the chest mass with no evidence of MIBG-avid disease elsewhere.
The extension assessment was negative (no hepatic nor cutaneous nor medullary metastases). We postponed the tumor biopsy because of dyspnea and oxygen dependence. Chemotherapy in association to intravenous corticosteroid therapy was initiated rapidly in an attempt to avoid neurological sequelae and invasive surgery. The child was started on chemotherapy (Caroplatin, Etoposide) with 4 cycles being given every 3 weeks. On 131I MIBG scan, there was increased radiotracer uptake in the chest mass with no evidence of MIBG-avid disease elsewhere (Figure 5).
Our patient was classified Stage 3 according to the International Neuroblastoma Staging System classification or L2 according to the International Neuroblastoma Risk Group classification [3, 4]. No microorganism was seen on gram exam and there was no growth on culture of the pleural liquid. He was gradually weaned to room air and Oxygen therapy was stopped one week after chemotherapy infusion and chest radiography improved. The MRI realized after 4 courses of chemotherapy showed a good response estimated to 90% of the initial volume (Figure 6).
Figure 6: MRI after 4 courses of chemotherapy: reduction of the mediastinal neuroblastoma (estimated to 90%).
The surgeon underwent posterolateral left thoracotomy through the left inter-costal space. A macroscopically complete excision was done. The operative findings included well encapsulated tissular lesion with multiple loculations. The post-operative recovery was uneventful. Chest X-ray showed well expanded lungs. The anatomopathological examination showed abundant Schwannian stroma (>50%) with Widespead calcification containing areas of mature neuroblasts within islands of neuropil. The exam was consistent with the diagnosis of mixed ganglioneuroblastoma (favourable histopronostic). The Myc-N was not amplified. Currently, the infant is asymptomatic, and his Chest X-ray is normal.
Discussion
Hemorrhagic spontaneous pleural effusion is a very rare condition in neonates and infants. In the majority of cases, it occurs only if there is some underlying lesion or a defect in the clotting mechanism. In fact, hemothorax most commonly occurs after chest trauma. In the other cases, it can be caused by generalized hemorrhagic diseases (associated to other bleeding sites), pleural-based vascular malformations such as hemangioma or pulmonary arteriovenous malformation, fistula pulmonary sequestration and intrathoracic neoplasms [1]. To our knowledge, fewer publications reported cases of neuroblastoma revealed by spontaneous hemothorax worldwide. Our case has normal coagulation study and there was no history of trauma. In such cases, further investigation should be done in order to identify the underlying cause, including malignant disease [5]. Some studies have shown that about half of pediatrics pleural effusion can be caused by pneumonia, followed by malignancies, renal disorders, trauma, and heart failure. The incidence of malignancy is about 5-10% of causes of pleural effusion in children [5]. Among these tumors, neuroblastoma is a rare etiology. It is an embryonic tumor arising anywhere throughout the sympathetic nervous system. It is located into the posterior mediastinum in 17% of cases. We have reported in the literature several situations of distress caused by thoracic neuroblastoma in which respiratory support is essential for the survival [1].
In general, treatment of hemothorax includes arterial catheter embolization or surgery with supportive therapy. In our case, a compressive hemothorax resulted in respiratory failure requiring thoracic drainage. However, since neuroblastoma is a chemosensitive tumor, rapid resolution of the hemothorax can be expected after effective chemotherapy [6]. In the present patient, massive hemothorax led to respiratory failure without circulatory failure. This is why conservative treatment based on chemotherapy without an invasive procedure was chosen. In fact, it can be difficult to determine the origin of the bleeding on thoracotomy. Our patient was classified Stage 3 or L2. According to the INSS, the presence of a malignant pleural effusion leads to upgrade tumor in extrathoracic neuroblastoma to stage 4 and to stage 2B in thoracic neuroblastoma like our patient [3]. According to the International Neuroblastoma Risk Group (INRG) Staging System, pleural effusion is not considered as an IDRF. It does not constitute metastatic disease unless they are remote from the body compartment of the primary tumor, even with malignant cells [4].
Concerning the prognosis, although pleural disease is associated with reduced survival rates in patients with metastatic neuroblastoma, the impact on outcome of isolated pleural effusion in case of locoregional disease like our patient, is not clear [7]. In the study by Gupta et al., the incidence of pleural effusion was 10.5% (31/295 patients) [2]. 7 of the 9 pleural swabs examined found malignant cells. These results are corroborated by those of Cowie et al. who reported that 3 out of 16 patients who underwent cytological analysis had positive results in all 3 cases [7]. Gupta et al. found no significant difference in the survival of patients with neuroblastoma who had pleural effusion and those who did not [2]. These constatations were also reported by Rashmi et al. who stipulated that the prognosis of thoracic neuroblastoma does not change whether or not there is an associated pleural effusion [8]. Our patient has been treated in the intermediate risk group since no metastases have been reported and the N-Myc was not amplified. Nevertheless, clinical and radiological surveillance is necessary to detect earlier an eventual relapse.
Conclusion
Spontaneous pleural effusions in children and particularly in neonates and infants require exploration to identify the underlying cause, including malignant disease like mediastinal neuroblastoma. The prognosis of non metastatic and N-Myc non amplified cases is good if the diagnosis is made reasonably promptly and the treatment adequate. In the present case, chemotherapy has been shown to be effective in controlling effusion without the use of invasive methods. To conclude, any infants with spontaneous hemorrhagic pleural effusion should be investigated further to ascertain the underlying cause. Conservative treatment in mediastinal neuroblastoma should be initially encouraged if possible since it is a chemosensitive tumor.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 15, Mar 2021Accepted: Fri 26, Mar 2021
Published: Fri 09, Apr 2021
Copyright
© 2023 Faten Fedhila Ben Ayed. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2021.04.03
Author Info
Faten Fedhila Ben Ayed S. Ben Ahmed A. Borgi S. Kbaier E. Jbebli F. Mezghani L. Lahmar S. Haddad W. Douira N. Ben Jaballah S. Jlidi S. Rhayem M. Khemiri
Corresponding Author
Faten Fedhila Ben AyedAssociate Professor in Pediatry, Pediatric Department A, Bechir Hamza Children’s Hospital of Tunis, University of Tunis El Manar, Tunis, Tunisia
Figures & Tables




References
1. Aaron BL, Doohen DJ (1970) Spontaneous hemothorax in the newborn another cause for respiratory distress. Ann Thorac Surg 3: 258-262. [Crossref]
2. Gupta H, Conrad J, Khoury JD, McGregor LM (2007) Significance of pleural effusion in neuroblastoma. Pediatr Blood Cancer 49: 906-908. [Crossref]
3. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V et al. (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11: 1466-1477. [Crossref]
4. Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G et al. (2009) The International Neuroblastoma Risk Group (INRG) Staging System: An INRG Task Force Report. J Clin Oncol 27: 298-303. [Crossref]
5. Efrati O, Barak A (2002) Pleural Effusions in the Pediatric Population. Pediatr Rev 23: 417-426. [Crossref]
6. Shiokawa N, Okamoto Y, Kodama Y, Nishikawa T, Tanabe T et al. (2016) Conservative treatment of massive hemothorax in a girl with neuroblastome. Pediatr Int 58: 1090-1092. [Crossref]
7. Cowie F, Corbett R, Pinkerton CR (1997) Lung involvement in neuroblastoma: Incidence and characteristics. Med Pediatr Oncol 28: 429-432. [Crossref]
8. Das RR, Sami A, Seth R, Nandan D, Kabra SK et al. (2014) Thoracic neuroblastoma presenting as recurrent empyema. Nat Med J India 27: 84-85. [Crossref]
