Sleeve Gastrectomy in a Patient with Obesity and Ehlers-Danlos Syndrome: A Case Report
A B S T R A C T
Ehlers-Danlos syndrome (EDS) is an inherited connective tissue disorder with a huge variety of signs and symptoms. Gastrointestinal manifestations may be present in up to 50% of patients. We here report bariatric surgery in a patient with EDS, focusing on management challenges, preoperative assessment and one-year outcome. A 56-year-old woman with hypermobility-type (HM) EDS, BMI 42,5 kg/m2 and hypertension underwent laparoscopic sleeve gastrectomy (LSG). She was uneventfully discharged on POD3. One-year after the operation her BMI was 28,3 kg/m2 and hypertension receded. Postoperative upper GI series (POD-60) did show neither reflux nor esophageal dysmotility. Bariatric surgery in patients with EDS can be challenging due to the potential risks of wound healing. Proper preoperative assessment and follow up should be strongly recommended.
Keywords
Obesity, bariatric surgery, Ehlers-Danlos syndrome, gastroesophageal reflux, sleeve gastrectomy
Background
Ehlers-Danlos syndrome (EDS) is described by the Ehlers-Danlos National Foundation as a “heterogeneous group of inheritable connective tissue disorders characterized by articular hypermobility, skin extensibility and tissue fragility [1]. The incidence of EDS is approximately 1 in 5000 births. Formerly divided EDS into 11 subgroups according to clinical phenotype, the latest classification, published in 1998, recognizes 6 EDS subtypes, based on clinical characteristics, such as joint laxity, vascular manifestations, kyphoscoliosis, arthrochalasia and dermatosparaxis, pattern of inheritance, molecular and biochemical findings [1]. The most common subtype is the hypermobility type (HM, formerly type III) which comprises 90% of all diagnosed EDS patients. The main manifestations of EDS include hyperextensible skin, atrophic scars, easy bruising, joint hypermobility and variable involvement of internal organs.
Gastrointestinal involvement is a well-known complication of EDS. Patients can show either organic issues such as hiatal hernia, visceroptosis, rectoceles and rectal prolapse or functional problems such as altered gut motility. The association between HM-EDS and GI symptoms was first described by Hakim and Grahaem [2]. They found that HM-EDS patients had a significant increase in GI symptoms compared to age and sex-matched controls (37% vs. 11%). The most common GI symptoms were nausea, abdominal pain, constipation and diarrhea. Direct evidence of the association between functional GI disorders and HM-EDS was firstly reported by a group of gastroenterologists [3]. The main upper GI complications are megaesophagus, esophageal, gastric or small bowel diverticula, hiatal hernia, gastric bleeding and ulcers, perforation or hematoma of the GI tract spontaneously or after surgery [4].
Case Report
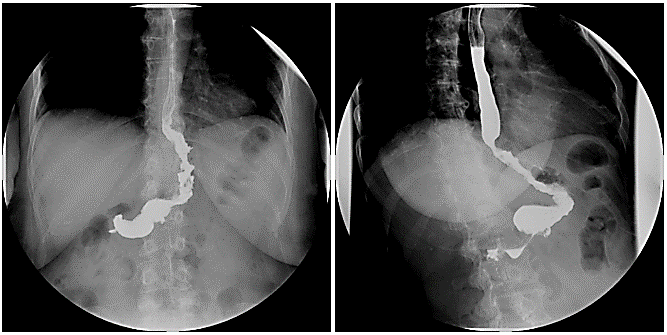
A 56-year-old woman with HM-EDS and BMI of 42,5 kg/m2 was referred to our Bariatric Unit for morbid obesity. Her only comorbidity was hypertension treated with diuretics and Ca channel blockers. She complained mild epigastric pain with episodic heartburn, without dysphagia. Pre-operatively, she underwent upper GI endoscopy that showed a small sliding hiatal hernia, chronic atrophic antral gastropathy with the absence of Helicobacter pylori, while a barium swallow (Figure 1) did not confirm the presence of hiatal hernia. No esophageal dysmotility but a regular esophageal clearance was evident.
Figure 1: Preoperative upper GI series.
She underwent LSG. She was uneventfully discharged on POD3. Two months later she had a control barium swallow that did not reveal any reflux/dysmotility and normal gastric emptying (Figure 2). At 6-month follow-up she was asymptomatic for heartburn/reflux symptoms and quit antihypertensive therapy; her BMI was 31,6 kg/m2/ and EWL% 61,9%. At 1-year follow-up she was still off antihypertensive drugs and PPI, BMI, EWL% and TWL were 28.3 kg/m2, 81% 34.5 kg, respectively.
Figure 2: Postoperative upper GI series.
Discussion
EDS may be neglected, and past medical and family history are key issues in the assessment of a patient candidate for bariatric surgery. The GI symptoms vary with subtypes of EDS. Nelson et al. described nausea (44%), heartburn (38%), vomiting (24,7%), bloating (17%) and dysphagia as the most common upper GI symptoms. Constipation (42,4%), IBD-like symptoms (30,3%) and diarrhea (22,5%) the most common lower GI symptoms [5].
Surgical procedures represent a real challenge when dealing with EDS patients, given the natural intrinsic frailty of treated tissues and their impaired “healing capacity”. Moreover, several cases of troublesome intraoperative bleeding and massive amounts of intraperitoneal adhesions have been reported [6]. General cautions should be considered during surgery in order to minimize surgical dissection, retraction and suturing. In fact, one of the most frequent issues is wound rupture even several weeks after surgery. Moreover, patients should be properly positioned in order to avoid joint dislocation and cutaneous injuries and particular care should be adopted during induction and awakening anaesthesia phases [7].
The most frequent early postoperative complications are related to anastomotic dehiscence and bleeding [8]. Iatrogenic oesophageal perforation after endoscopic procedures is reported as more frequent in EDS with predominant vascular type, less in the remaining subtypes [9]. In our case, no complication occurred. Considering late complications, spontaneous oesophageal perforation, oesophageal diverticula, megaesophagus, gastric atony, megaduodenum, small bowel dilation, megacolon and delayed gastric emptying are reported [1, 10, 11].
In our case we chose LSG taking into consideration the clinical conditions and past medical history of the patient. This operation is considered less invasive than Roux-en-Y gastric bypass, involving less tissue manipulation, no anastomosis but only a single long staple line. Moreover, the patient had no history of type 2 diabetes, and obstructive sleep apnoea syndrome and matched the selection criteria for bariatric surgery [12].
Despite the potential challenges posed by surgery on EDS patients, 1-year outcome was very satisfactory, as for comorbidity resolution (hypertension) and weight loss. Further research, longer follow-up periods and more cases are needed to confirm the safety and efficacy of LSG on EDS patients with obesity.
Conclusion
Bariatric surgery on patients with EDS can be challenging and stimulating at the same time. A patient with EDS and morbid obesity may increase the engagement and commitment of the multidisciplinary team, well aware of the potential surgical risks but less aware of the management of EDS-related surgical complications. Accordingly, a strict follow-up policy is advisable in order to assess any eventual postoperative issues.
Article Info
Article Type
Case ReportPublication history
Received: Thu 25, Jun 2020Accepted: Thu 09, Jul 2020
Published: Mon 20, Jul 2020
Copyright
© 2023 Amanda Belluzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GSCR.2020.01.08
Author Info
Corresponding Author
Amanda BelluzziBariatric Unit-Padova University Hospital, Italy
Figures & Tables

References
- P Beighton, A De Paepe, B Steinmann, P Tsipouras, R J Wenstrup (1998) Ehlers-Danlos Syndromes: revise nosology,Villefranche,1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 77: 31-37. [Crossref]
- A J Hakim, R Grahame (2004) Non-musculoskeletal symptoms in joint hypermobility syndrome. Indirect evidence fora autonomic disfunction? Rheumatology (Oxford) 43: 1194-1195. [Crossref]
- N Zarate, A D Farmer, R Grahame, S D Mohammed, C H Knowles et al. (2009) Unexplain gastrointestinal symptoms and joint hypermobility: Is connective tissue the missing link? Neurogastroenterol Motil 22: 252-e78. [Crossref]
- Sandy Fogel (2013) Surgical failures: is it the surgeon or the patient? The all too often missed diagnosis of Ehlers-Danlos Syndrome. Am Surg 79: 608-613. [Crossref]
- A D Nelson, M A Mouchli, N Valentin, D Deyle, P Pichurin et al. (2015) Ehler Danlos sybdrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterol Motil 27: 1657-1666. [Crossref]
- J A Solomon, L Abrams, G R Lichtenstein (1996) GI manifestations of Ehlers Danlos Syndromes. Am J Gastroenterol 91: 2282-2288. [Crossref]
- Jakob Burcharth, Jacob Rosenberg (2012) Gastrointestinal Surgery and Related Complications in Patients with Ehlers-Danlos Syndrome: A Systematic Review. Dig Surg 29: 349-357 [Crossref]
- R K Freeman, J Swegle, M J Sise (1996) The surgical complications of Ehlers-Danlos Syndrome. AM Surg 62: 869-873. [Crossref]
- Saikiran M Kilaru, Kenneth J Mukamal, Judy W Nee, Sveta S Oza, Anthony J Lembo et al. (2019) Safety of Endoscopy in Heritable Connective Tissue Disorders. Am J Gastroenterol 114: 1343-1345. [Crossref]
- Marie-Louise Kulas Søborg, Julie Leganger, Jacob Rosenberg, Jakob Burcharth (2017) Increased Need for Gastrointestinal Surgery and Increased Risk of Surgery-Related Complications in Patients with Ehlers-Danlos Syndrome: A Systematic Review. Dig Surg 34: 161-170. [Crossref]
- R L McEntyre, J G Raffensperger (1977) Surgical complications of Ehlers-Danlos syndrome in children. J Pediatr Surg 12: 531-535. [Crossref]
- National Institute for Health and Care Excellence (2014) Obesity: Identificaation, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. Clinical Guideline CG 189. London: NICE. [Crossref]
