Single-Dose Pembrolizumab Achieving Remission in Patient with Refractory Hodgkin’s Lymphoma
A B S T R A C T
Background: Hodgkin's lymphoma (HL) is a hematopoietic tumor that is distinguished by the presence of Reed-Sternberg cells in a background of inflammatory cells. Advancements in cancer research have driven significant motions in cancer-related overall survival outcomes, which has led to higher rates of developing secondary neoplasms.
Case Presentation: A 22-year-old-woman with a past medical history of non-Hodgkin's lymphoma (NHL) who presents to the hospital for respiratory manifestations and unintentional weight loss. Chest Computed Tomography (CT) scan showed left axillary lymphadenopathy; biopsy proved nodular sclerosing stage IVB HL. The patient started an anthracycline free regimen, but unfortunately, she developed an acute kidney injury, and thus, cisplatin was discontinued and switched to brentuximab therapy with hemodialysis. After the second cycle of salvage brentuximab therapy, the patient was admitted to the hospital for post obstructive pneumonia-causing acute hypoxic respiratory failure, and the decision was made to start the patient on immunotherapy with pembrolizumab. However, during administering pembrolizumab, the patient developed acute respiratory distress, and she ended up requiring emergent intubation and was admitted to the medical intensive care unit. Therefore, it was decided that pembrolizumab will not be given again. The patient later stabilized, and surprisingly, upon follow-up, the patient was found to have negative fluorodeoxyglucose (FDG) PET/CT scan, which indicates the remission of her HL.
Conclusion: Recognize the critical role of the anti-programmed cell death protein-1 monoclonal antibodies in patients with chemo-resistant Hodgkin's Lymphoma (HL).
Keywords
Pembrolizumab, refractory hodgkin’s lymphoma, non-hodgkin’s lymphoma
Background
The improved survival rate of a patient with non-Hodgkin’s lymphoma (NHL) has led to the rise in long term treatment-related complications, particularly secondary cancers, with risk expected for up to 20 years after treatment [1]. Hodgkin’s lymphoma (HL) is one of the most frequent secondary cancers, and the risk of acquiring it suggested to be higher in people treated with both chemotherapy and/or radiation. Both HL and refractory HL were effectively treated with pembrolizumab, which is an anti-programmed cell death protein-1 (PD-1) monoclonal antibody that targets the PD-1 that is found to be overexpressed by the altered chromosome 9 [2, 3]. The objective of this report is to identify the role of the anti-programmed cell death protein-1 monoclonal antibodies, pembrolizumab, in patients with chemo-resistant HL. In our report, we present a unique case where only one dose of pembrolizumab achieved remission in a refractory HL that developed ten years post complete remission of NHL.
Case Report
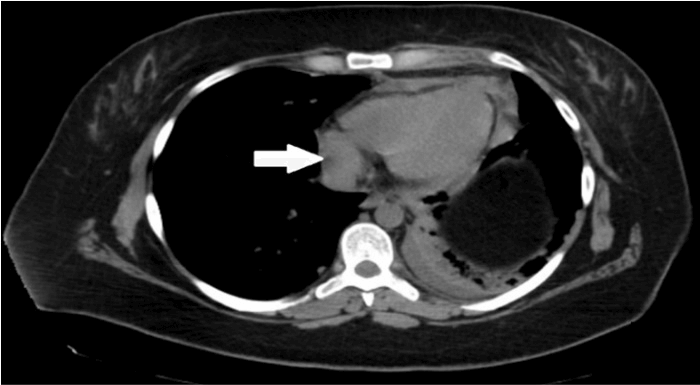
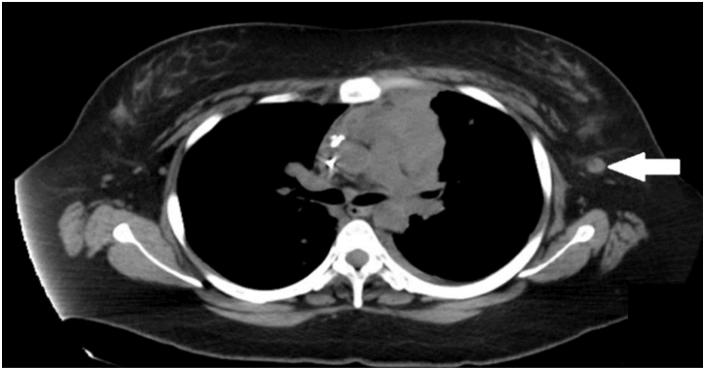
A 22-year-old-woman with a past medical history of NHL was admitted to the hospital for worsening dyspnea and unintentional weight loss. Her NHL was diagnosed at the age of 13 years, and complete remission was achieved with chemoradiotherapy to the mediastinum. The examination was relevant for dullness to percussion with reduced air entry at both lung bases and a palpable hard-Left axillary lymphadenopathy. Labs were pertinent for white blood cell (WBC) count 7.8 × 109/L, neutrophils 50%, lymphocytes 5%, hemoglobin 11.3 g/dl, platelets of 337 × 109/L, BUN 9 mg/dL, creatinine 0.57 mg/dL, sodium 145 mEq/L, potassium 4.1 mEq/L, chloride 108 mEq/L, CO2 23 mEq/L, glucose 87 mg/dL. Chest Computed Tomography (CT) scan revealed pericardial lymphadenopathy with effusion, extensive mediastinal lymph node conglomeration measuring 5.7 x 4.6 cm and left axillary lymph node with surrounding inflammatory stranding which raised suspicion for recurrence of lymphoma (Figures 1 & 2).
Figure 1: Chest CT scan showing pericardial lymphadenopathy the largest measuring 1.4 cm in the short axis (arrow).
Figure 2: Chest CT scan showing 1.1 cm left axillary lymph node with surrounding inflammatory stranding (arrow).
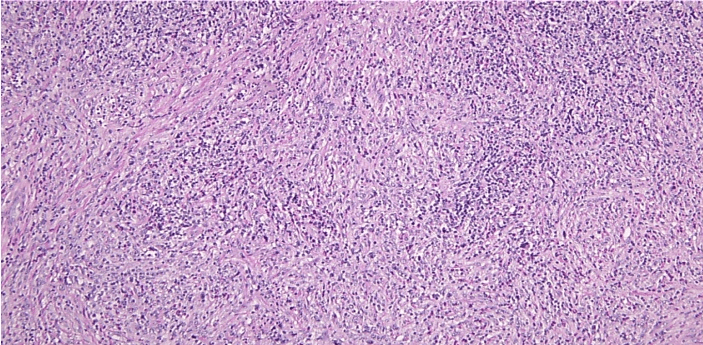
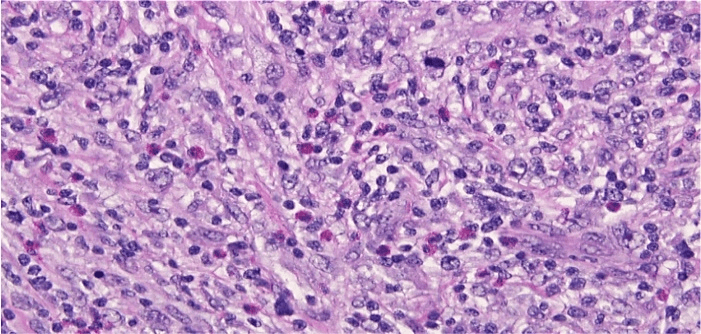
Left axillary lymph node biopsy reported stage IVB HL nodular sclerosing type (Figures 3-9). Anthracycline-free regimen, including gemcitabine, cisplatin, and decadron, was started for the patient. Pleural effusion later resolved, but during the course of treatment, the patient developed an acute kidney injury (AKI) with a BUN of 21 mg/dL, creatinine 3.07 mg/dL, and thus cisplatin was discontinued and switched to brentuximab and patient was continued on treatment with hemodialysis. After the 2nd cycle of salvage brentuximab therapy, the patient was admitted to the hospital for post obstructive pneumonia-causing acute hypoxic respiratory failure. CT pulmonary angiography (CTPA) was negative for pulmonary embolism, and a pulmonary biopsy was performed, which showed pulmonary involvement of HL. In addition, the patient was given a course of cefepime for progressive leukopenia where WBC count reached to 1.5 × 109/L with neutropenia where neutrophils reached only 20%.
Figure 3: Left axillary lymph node biopsy specimen at x 100 magnification showing effacement of architecture and nodule formation by collagenous bands and extranodular extension of neoplastic process.
Figure 4: Left axillary lymph node biopsy specimen at x 400 magnification showing cellular infiltrate within the nodular areas which is composed of many nodular Hodgkin’s cells, lacunar cells and scattered classical Reed Sternberg cells in a mixed background of lymphocytes, plasma cells, significant number of eosinophils with rare neutrophils and macrophages.
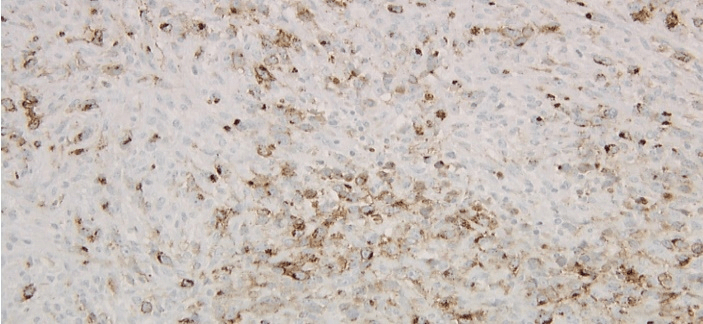
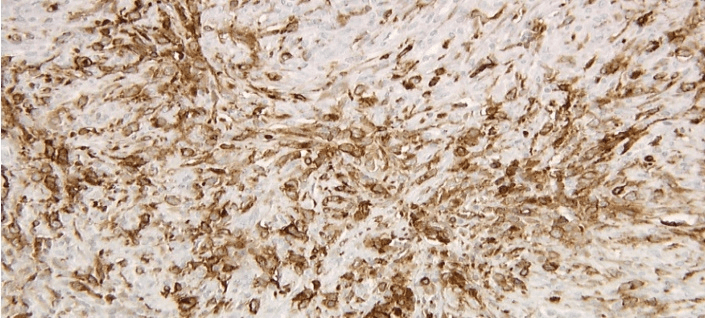
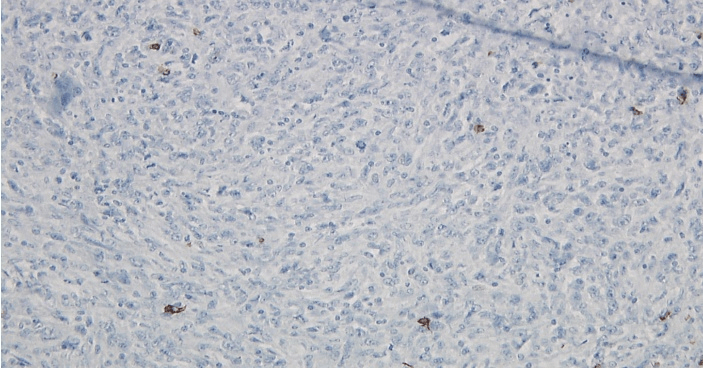
Figure 5: Left axillary lymph node biopsy specimen immunoperoxidase CD15 positive stain.
Figure 6: Left axillary lymph node biopsy specimen immunoperoxidase CD30 positive stain.
Despite that, the patient continued to complain of worsening dyspnea, and repeat CTPE showed pulmonary embolism, and the patient was placed on anticoagulation. The decision was made to start the patient on immunotherapy pembrolizumab. About 20 minutes after starting the new cycle of pembrolizumab; the patient developed shortness of breath, stridor, wheezing and was getting somnolent without skin reactions, and thus, the patient was given decadron, racemic epinephrine with minimal improvement and thus, she ended up requiring emergent intubation and was admitted to medical intensive care unit (MICU). It is thought that this was likely due to adverse reaction to pembrolizumab, and given this, it was decided that it will not be given again. Given the patient’s overall poor prognosis due to her advanced lymphoma, hospice care continued to be offered, however, was declined by the patient and family.
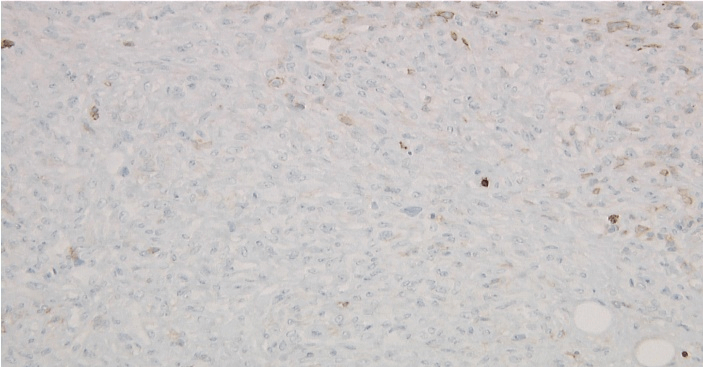
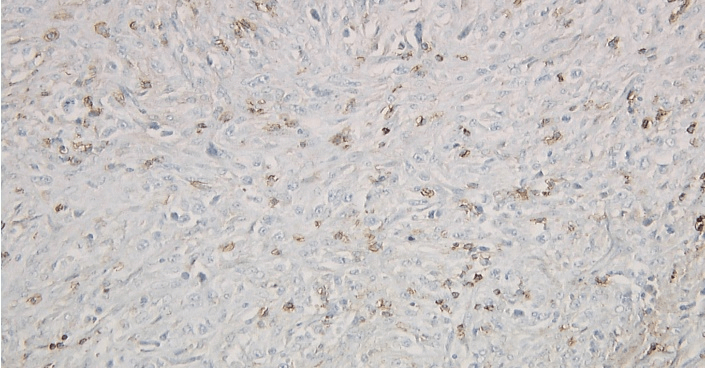
Figure 7: Left axillary lymph node biopsy specimen immunoperoxidase LCA negative stain.
Figure 8: Left axillary lymph node biopsy specimen immunoperoxidase CD45 RO negative stain.
Figure 9: Left axillary lymph node biopsy specimen immunoperoxidase CD20 negative stain.
The patient later stabilized, her kidney function recovered and no longer required dialysis, and thus, she was transferred from MICU to the internal medicine unit, and later discharged home. Surprisingly, upon follow-up, three weeks later, the patient was found to have negative fluorodeoxyglucose (FDG) PET/CT scan, which indicates the remission of her HL. The patient was later started again on brentuximab and remained in remission after 17 months of follow-up.
Discussion
Chemotherapy and/or radiation therapy are the main therapy for NHL. They have led to improved survival in these patients, and consequently, 1.88-fold increased risk of secondary malignancies in patients who were cured of this disease compared to the general population [4]. Hodgkin lymphoma is a curable lymphoma that tends to affect young patients and is one of the secondary cancers that might occur after successful remission of NHL [5]. The Ann Arbor classification is most often used to classify HL as follows: Stage I when a single lymph node or a single extra-nodal region involved. Stage II, when two or more lymph node regions on the same side of the diaphragm are involved. Stage III, when bilateral lymph node areas of the diaphragm are involved. Stage IV signifies the disseminated involvement of extra-nodal organs. Furthermore, each stage is subdivided into A or B based on the clinical symptoms. NHL is distinguished from HL by lymph node biopsy [6].
The tumor microenvironment is rich in T cells and non-malignant B cells. PD-1 is expressed by B and T lymphocytes: natural killer (NK) cells and monocytes and has two ligands, programmed death-ligand 1 (PD-L1) and PD-L2, that bind to PD-1 and induce downregulation and apoptosis in most PD-1+ leukocytes [7]. Tumor cells overexpress PD-1 ligand, which interacts with PD-1 on the effector T-cell and suppresses the immune effector T-cell activity and suppresses the patient immunity, hence helping the tumor cell evade the immune system. HL has up-regulated program death ligand 1 (PD-L1) in >95% of cases, creating a state of so-called “T cell exhaustion,” which can be reversed with immune checkpoint-inhibitor blockade [8]. Pembrolizumab, which is an anti-PD-1 monoclonal antibody, has demonstrated a high objective response rate (ORR) in chemo resistant HL. Ongoing studies are exploring combining pembrolizumab with other biologic agents in this group of patients, which is found that it might lead to increase chemo-sensitivity and produce an additive or even synergistic effect [9-11]. Uniquely, our patient achieved remission of refractory HL with a single cycle of pembrolizumab.
Conclusion
Anti-PD-L1 treatment is effective in achieving remission in patients with relapsed or refractory HL. This unique case of refractory HL remission achieved with a single dose of pembrolizumab implies a significant role of anti-PD-L1 in HL management. Further research is needed to stratify response to treatment further, whether single therapy or combination therapy and outcomes based on patient characteristics and tumor burden to help thoroughly understand this therapy and predict its outcomes.
Disclosures
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 30, Mar 2020Accepted: Thu 09, Apr 2020
Published: Mon 20, Apr 2020
Copyright
© 2023 Dawood Findakly. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.03
Author Info
Carmel Moazez Dawood Findakly Surabhi Amar
Corresponding Author
Dawood FindaklyCreighton University School of Medicine, Omaha, Nebraska, USA
Figures & Tables
References
- Oran B, Weisdorf DJ (2012) Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 97: 1916-1924. [Crossref]
- Thompson CA, Mauck K, Havyer R, Bhagra A, Kalsi H et al. (2011) Care of the adult Hodgkin lymphoma survivor. Am J Med 124: 1106-1112. [Crossref]
- Wang Y, Nowakowski GS, Wang ML, Ansell SM (2018) Advances in CD30- and PD-1-targeted therapies for classical Hodgkin lymphoma. J Hematol Oncol 11: 57. [Crossref]
- Dracham CB, Shankar A, Madan R (2018) Radiation induced secondary malignancies: a review article. Radiation Oncol J 36: 85-94. [Crossref]
- Lisik Habib M, Czernek U, Dębska Szmich S, Krakowska M, Kubicka Wołkowska J et al. (2015) Secondary cancer in a survivor of Hodgkin's lymphoma: A case report and review of the literature. Oncol Lett 9: 964-966. [Crossref]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH et al. (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32: 3059-3068. [Crossref]
- Jiang X, Wang J, Deng X, Xiong F, Ge J et al. (2019) Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 18: 10. [Crossref]
- Dong Y, Sun Q, Zhang X (2017) PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 8: 2171-2186. [Crossref]
- Shindiapina P, Alinari L (2018) Pembrolizumab and its role in relapsed/refractory classical Hodgkin's lymphoma: evidence to date and clinical utility. Ther Adv Hematol 9: 89-105. [Crossref]
- Christian Bailly, Xavier Thuru, Bruno Quesnel (2020) Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer 2: zcaa002.
- Wu X, Li Y, Liu X, Chen C, Harrington SM et al. (2018) Targeting B7-H1 (PD-L1) sensitizes cancer cells to chemotherapy. Heliyon 4: e01039. [Crossref]
