Journals
Case report: Should associating liver partition and portal vein ligation for staged hepatectomy be used for hepatocellular carcinomas with advanced portal vein tumour thrombi?
A B S T R A C T
We describe the case of a hepatocellular carcinoma (HCC) patient with portal vein tumour thrombus (PVTT) and middle hepatic vein (MHV) invasion undergoing ALPPS operation. Unfortunately, the patient finally died because of serious intestinal paralysis and secondary infection. We explored some important issues about patient selection of HCC patients with PVTT and MHV and the correlation between the function and regeneration volume of future liver remnant in such patients.
K E Y W O R D S
Liver cancer, tumour thrombus, middle hepatic vein invasion
I N T R O D U C T I O N
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer [1]. The invasion of the portal vein, which indicates a poor prognosis, occurs in approximately 12.5-39.7% patients with HCC [2, 3]. Simultaneously, due to the limited future liver remnant (FLR) and the further progression of the tumour thrombus, vascular invasion is always a substantial challenge in the treatment of HCC. Although hepatectomy may provide a treatment for such patients with portal vein tumour thrombi, its application is very limited due to poor outcomes [4]. According to the National Comprehensive Cancer Network (NCCN) guidelines, the risk of liver failure after hepatectomy will increase when the FLR is less than 30% of the total volume of the normal liver and less than 40% in fibrotic patients [5]. All the time, transcatheter arterial chemoembolization (TACE) has been regarded as an effective method for unresectable tumours because of an existing contraindication [6]. However, TACE is not regarded as the best indication for advanced portal vein tumour thrombus (PVTT) patients according to Cheng [7].
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), introduced by De Santibaoes E in 2012, was characterized by the rapid hypertrophy of the FLR through the ligation of the portal vein and liver dissection [8]. To some extent, this method can localize the scope of tumour invasion through division. However, the feasibility of this method for PVTT is still unclear, and currently no reports have described its application for both portal vein and middle hepatic vein (MHV) invasion.
Case Presentation
A 57-year-old woman presented with a small HCC. She had previously undergone ablation of segment IV/ VIII one year ago. This time, she was hospitalized for tumour recurrence within segment IV/ VIII. A magnetic resonance imaging (MRI) scan revealed multiple tumour nodules in segment VI / VIII, the largest of which was approximately 1.6 cm in diameter. The tumour had invaded the right anterior branch of the portal vein and formed a PVTT (Figure 1). The tumour-free liver existed chronic hepatitis. The hepatic hilar lymph node was considered a metastasis. Laboratory examinations indicated that the patient had no hypersplenism with an “A” of Child-Pugh level. Her model for end-stage liver disease (MELD) score was 9, and alpha-fetoprotein (AFP) level was more than 1000ng/mL. The patient had a 6-year hepatitis B virus (HBV) infection history in the past and had regularly received entecavir treatment, the quantity of HBV-DNA was undetectable with an secular standard method. We performed TACE combined with sorafenib according to the BCLC system to inhibit the progression of the tumour and provide an opportunity for subsequent hepatectomy. Although the tumour thrombus had been fully embolized by TACE, it still progressed rapidly, developed in the main track of the portal vein and finally invaded the branch of the MHV one month later (Figure 2, Figure 3). These results were not expected and indicated the highly invasive characteristic of the tumour. A computed tomography (CT) volume scan revealed that the FLR was 308.77mL. If an extended right lobe hepatectomy can be performed for this patient, we calculated the ratio of the FLR to the standard total liver volume (sTLV). It was only 28.5%, which is insufficient for safe hepatectomy. Portal vein embolism (PVE) and portal vein ligation (PVL) could also not be performed due to the long-time interval and the slow regeneration rate of FLR. Finally, we selected ALPPS for this patient.
I First Operation
After careful evaluation, we implemented the first-stage operation, and we found multiple tumours in the right lobe of the liver, partial tumour nodules could be seen on the surface of segment Iva, and the remaining left lobe was small. Simultaneously, multiple hard lymph nodules were found in the duodenal ligament. The right branch of the portal vein, hepatic artery and bile duct were separated and labelled with various coloured ribbons. Then, we ligatured the right branch of the portal vein under the guidance of intraoperative ultrasonography. The separation line ranged from the right part of segment IVb to the left of segment IVa. A dripping electric knife was used for the liver transaction. The fast-frozen pathology showed the hepatic hilar lymph node a metastatic carcinoma accompanied with necrosis. Therefore, lymph node dissection was performed in the duodenal ligament.
The patient exhibited a good recovery and got out of bed 3 days after the operation. No bile leakage occurred after the operation. Her liver function was nearly normal. A postoperative CT scan indicated the tumour was localized to the right liver lobe, and no metastatic lesions were observed in the FLR. A month later, the regeneration volume of the FLR was 147.3mL, and the kinetic growth rate (KGR) was 5.25mL per day, the ratio of the FLR to sTLV exceeded 30% (Table 1). Therefore, a second-stage operation was performed.
II Second Operation
Although minor ascite was found before the operation, the left liver lobe was larger and reached the requirement for second-stage operation. We released the adhesion around the portal vein, separated the right branch of hepatic artery and right bile duct, and ligated them. We performed an extended right hepatectomy depending on the separation line during the first operation. Meanwhile, the metastatic heart-septal lymph node (Figure 4) was dissected via video-assisted thoracic surgery. The total operation time was approximately 6 hours, and nearly 400 mL of blood was lost during the operation. The postoperative pathology indicated a differentiated HCC with vascular invasion.
The patient recovered well at the early stage after operation. However, she experienced serious intestinal paralysis and secondary infection 5 days later. And subsequently multiple organ dysfunction syndrome (MODS) occurred, finally she died when all rescue measures proved ineffectual.
Figures & Tables
Table 1: Hyperplasia data after the first-stage operation
|
Pre-operation |
POD 7 |
POD 14 |
POD 21 |
POD 28 |
|
|
FLR (mL) |
308.77 |
362 |
370 |
379.4 |
455.8 |
|
FLR/sTLV(%) |
28.5 |
33.5 |
34.2 |
35.1 |
42.1 |
|
DH (mL) |
|
53.23 |
61.23 |
70.73 |
147.3 |
|
KGR (mL)
|
|
7.6 |
4.37 |
3.37 |
5.25 |
POD, postoperative day; FLR, future liver remnant; sTLV, standard total liver volume
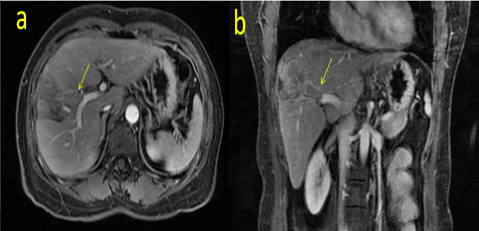
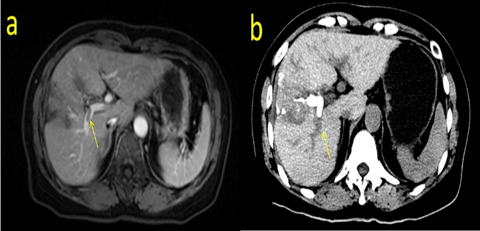
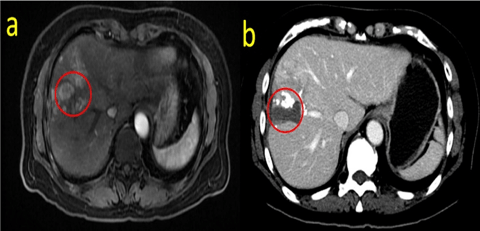

Figure legends
Fig. 1: MRI scan before TACE showing the PVTT inside the right anterior branch of the portal vein. (a shows the cross section, b represents the coronary position)
Fig. 2: MRI and enhanced CT scan after TACE showing the PVTT adjacent to the right branch of the portal vein. (a shows the MRI scan, b shows the enhanced CT scan)
Fig. 3: MRI scan showing the HCC invading the distal branch of the MHV. (a shows the T2 phase, b represents the portal vein phase)
Fig. 4: MRI scan showing right side heart septal lymph node metastasis at POD 7
Discussion
HCC is the most common primary malignant tumour worldwide [9]. The vascular invasion of HCC, including PVTT or MHV invasion, is one of the important factors affecting the prognoses for advanced HCC patients. Generally PVTT is very common when diagnosis with HCC, and this is a dangerous signal. It is reported that the 3-year and 5-year survival rate for HCC patients with PVTT is 18.9% and 8.3% respectively [10,11]. Treatment for such patients is still controversial, although sorafenib can have certain curative effect according to the BCLC [12]. Kashiwazaki M reported that the overall median survival time of patients who received surgery was 344.5 days, which is much longer than 67 days for those who do not accept surgery [13]. On the other hand, Qi Zhou reported the 1- and 3-year survival rate of patients with PVTT who receive conservative therapy were only 12% and 4% respectively [14].
According to Cheng’s tumour thrombus classification system, this patient in the report belongs to type II [7]. The malignant and invasive characteristics of HCC were obviously reflected in this case. Initially, the extended right hepatectomy was limited due to the insufficient volume of the FLR, then neither TACE nor PVE were planned in case of PVTT progression or slow regeneration. Finally, we chose ALPPS for this patient since liver transaction at first stage could limit the progression of PVTT and development of MHV tumour thrombus.
ALPPS is a new hepatectomy method which is different from conventional two staged hepatectomy (TSH) [8]. Although the regeneration rate of ALPPS is significantly higher than TSH, meanwhile mortality and complication rate remain higher in ALPPS [15-17]. It was reported that their 90-day mortality in ALPPS was 11%, and patients undergoing ALPPS often experienced more complications than TSH [18]. However, the effectiveness and prognosis of this procedure still remain controversial due to the lack of prospective randomized controlled trials.
After the first stage of ALPPS, the patient suffered an insufficient FLR regeneration. Although a rapid FLR regenerative rate was observed during the first week after the operation, the average KGR was not high. Previous studies reported that the sufficient regeneration incidence of FLR in TSH was 0-20.7% [15, 17, 19, 20]. In fact, regeneration in ALPPS was also insufficient. For this phenomenon, some reasons for may be considered:
- Most patients have a long HBV infection history which leads to chronic inflammation and final cirrhosis, hence liver regeneration is seriously affected.
- The haemodynamic change of portal vein is the main factor which affects regeneration of ALPPS.
- Although ultimate regeneration rate exceeded 30%, low KGR indicated a poor quality of regeneration. Indeed, the relationship between liver function and the regeneration volume still remains unclear. Currently, no relevant reports had examined the occurrence rate of tumour in patients who undergo ALPPS.
- Although the ligation of portal vein of ALPPS can increase arterial blood flow caused by hepatic arterial buffer effect, hence promoting the growth of the HCC. The indicator of cell proliferation, ki-67, was higher before the operation, which may explain the above augments in turn [21, 22]. Simultaneously, for this patient, no tumour progression occurred in the right liver lobe according to CT scan. The first-stage operation, including liver partition and portal vein ligation, was able to localize the primary tumour.
Generally speaking, the 90-day mortality and complication rates associated with ALPPS are higher than those in TSH. One reason is the hypertransfusion of portal vein, which has been reported to cause high mortality and more complications after surgery [23]. Dipok Kumar Dhar pointed out that early regeneration was associated with significantly increasing portal pressure in the ALPPS model of rats [24]. E. Vicente viewed small-for-size syndrome of two patients who received first-stage operation, and one of them finally died because of liver failure. And pathological examination revealed an important sinusoidal dilatation, which maybe a common pathological change after first-stage ALPPS operation [25]. Similarly, although PVE or PVL could stimulate liver regeneration, they may also lead to liver failure due to the excessive portal vein blood [26]. Additionally, Kristopher pointed out that lower KGR indicating higher risk of liver insufficiency in patients accepting PVE [15]. So, it is necessary to evaluate the FLR function before operation and establish standards assessing the relationship between increased regeneration volume and FLR function.
According to our knowledge, this is the first report for HCC patient with PVTT and MHV invasion who received ALPPS. We also firstly reported such case of insufficient regeneration and tumour progression during the time interval of ALPPS. Preoperative status of FLR may impair liver regeneration efficiency, it is necessary to evaluate the function of FLR before second-stage operation.
Conflicts of Interest
There is no conflict of interest to disclose.
Article Info
Article Type
Research ArticlePublication history
Received 23 March, 2018Accepted 12 April, 2018
Published 20 April, 2018
Copyright
© 2018 Ruiyun Xu and Zheng Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository. All rights reserved.Author Info
Corresponding author
Ruiyun Xu and Zheng ZhouDepartment of Hepatobiliary Surgery, Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630
Figures & Tables
Table 1: Hyperplasia data after the first-stage operation
|
Pre-operation |
POD 7 |
POD 14 |
POD 21 |
POD 28 |
|
|
FLR (mL) |
308.77 |
362 |
370 |
379.4 |
455.8 |
|
FLR/sTLV(%) |
28.5 |
33.5 |
34.2 |
35.1 |
42.1 |
|
DH (mL) |
|
53.23 |
61.23 |
70.73 |
147.3 |
|
KGR (mL)
|
|
7.6 |
4.37 |
3.37 |
5.25 |
POD, postoperative day; FLR, future liver remnant; sTLV, standard total liver volume




Figure legends
Fig. 1: MRI scan before TACE showing the PVTT inside the right anterior branch of the portal vein. (a shows the cross section, b represents the coronary position)
Fig. 2: MRI and enhanced CT scan after TACE showing the PVTT adjacent to the right branch of the portal vein. (a shows the MRI scan, b shows the enhanced CT scan)
Fig. 3: MRI scan showing the HCC invading the distal branch of the MHV. (a shows the T2 phase, b represents the portal vein phase)
Fig. 4: MRI scan showing right side heart septal lymph node metastasis at POD 7
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108. [Crossref]
2. Minagawa M, Makuuchi M (2006) Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 12: 7561-7567. [Crossref]
3. Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, et al. (2007) Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology 54: 499-502. [Crossref]
4. Li N, Feng S, Xue J, Wei XB, Shi J, et al. (2016) Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford) 18: 549-556. [Crossref]
5. Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, et al. (2009) NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 7: 350-391. [Crossref]
6. Zhang ZM, Lai ECH, Zhang C, Yu HW, Liu Z, et al. (2015) The strategies for treating primary hepatocellular carcinoma withportal vein tumor thrombus. Int J Surg 20: 8-16. [Crossref]
7. Shi J, Lai EC, Li N, Guo WX, Xue J, et al. (2011) A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci 18: 74-80. [Crossref]
8. De SE, Alvarez FA, Ardiles V (2012) How to avoid postoperative liver failure: a novel method. World J Surg 36: 125-128. [Crossref]
9. Llovet JM, Burroughs A, Bruix J (2014) Hepatocellular carcinoma. Wiener Medizinische Wochenschrift 3: 55.
10. Cheng S, Chen M, Cai J (2017) Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget 8: 8867-8876. [Crossref]
11. Takizawa D, Kakizaki S, Sohara N, Sato K, Takagi H, et al. (2007) Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: Clinical Characteristics, Prognosis, and Patient Survival Analysis. Dig Dis Sci 52: 3290-3295. [Crossref]
12. Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease 19: 329-338. [Crossref]
13. Kashiwazaki M, Hama N, Takiuchi D, Noguchi K, Hata T, et al. (2013) [Resection and postoperative multidisciplinary treatment for hepatocellular carcinoma with massive portal venous tumor thrombus-a single-center experience]. Gan To Kagaku Ryoho 40: 1675-1677. [Crossref]
14. Zhou Q, Wang Y, Zhou X, Peng B, Yang J, et al. (2011) Prognostic analysis for treatment modalities in hepatocellular carcinomas with portal vein tumor thrombi. Asian Pac J Cancer Prev 12: 2847-2850. [Crossref]
15. Croome KP, Hernandez‐Alejandro R, Parker M, Heimbach J, Rosen C, et al. (2015) Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford) 17: 477- 484. [Crossref]
16. Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, et al. (2016) Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 159: 1289-1298. [Crossref]
17. Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, et al. (2014) ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 38: 1510-1519. [Crossref]
18. Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, et al. (2015) Systematic Review and Meta-Analysis of Feasibility, Safety, and Efficacy of a Novel Procedure: Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. Ann Surg Oncol 22: 3109-3120. [Crossref]
19. Ratti F, Schadde E, Masetti M, Massani M, Zanello M, et al. (2015) Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol 22: 1933-1942. [Crossref]
20. Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, et al. (2013) Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume including a comparison to the ALPPS approach. J Am Coll Surg 217: 126-133. [Crossref]
21. Lautt WW (1985) Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol 249: 549-556. [Crossref]
22. Fukami Y, Kurumiya Y, Kobayashi S (2014) Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): an analysis of tumor activity. Updates Surg 66: 223-225. [Crossref]
23. Truant S, Scatton O, Dokmak S, et al. (2015) Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol 41: 674-682. [Crossref]
24. Dhar DK, Mohammad GH, Vyas S, Broering DC, Malago M (2015) A novel rat model of liver regeneration: possible role of cytokine induced neutrophil chemoattractant-1 in augmented liver regeneration. Ann Surg Innov Res 9: 11. [Crossref]
25. Vicente E, Quijano Y, Ielpo B, Duran H, Diaz E, et al. (2015) Is "small for size syndrome" a relatively new complication after the ALPPS procedure? Updates Surg 67: 273-278. [Crossref]
26. Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, et al. (2016) Small for Size and Flow (SFSF) syndrome: An alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol 40: 267-275. [Crossref]
