Risk Factors for the Recurrence of Basal Cell Carcinomas with Special Remarks on the R-Status
A B S T R A C T
Background: Surgical excision remains the gold standard in the treatment of operable basal cell carcinoma (BCC). According to national guidelines, total resection with tumor-free margins should always be achieved in order to avoid recurrence. The aim of this study was to investigate surgical, histological as well as tumor-specific risk factors for the recurrence of BCC of the head and neck.
Materials and Methods: In a retrospective analysis, patients who were operated on because of a BCC of the head and neck between 2011 and 2014 were included. Epidemiological as well as therapeutic and histological data were collected and investigated concerning the recurrence of the tumor. Follow-up data were controlled for recurrence.
Results: 141 patients with a mean age of 72.9 years were included in this study. Most frequent tumor localizations were the nose and temple, and most of the BCC were nodular. Altogether, 8.6% of the patients showed a recurrence. In the chi-squared analysis, R-status or the resection margin did not correlate with the risk of BCC recurrence at the same localization. In contrast, the significant risk factors for recurrence were the tumor localization, size, and histological subtype.
Conclusion: Incomplete total resection (R1) of a BCC does not inevitably lead to a recurrence. A reason therefore might be immune-mediated regression, which can be seen in other tumor entities as well. However, a total resection should still always be the aim for surgical therapy, especially for cases with high risk for recurrence.
Keywords
Basal cell carcinoma, surgical oncology, head and neck surgery, tumor recurrence
Introduction
With approximately well over 200,000 new diagnoses each year in Germany, non-melanoma skin cancer (NMSC) remains the most common malignancy of Caucasians, with the vast majority of these cases being basal cell carcinomas (BCC). This entity, in fact, underwent a threefold increase of its incidence within the last three decades, and despite screening campaigns and growing awareness towards skin cancer in the population, it is assumed that the actual number is even higher due to possible undiagnosed cases [1, 2].
Since the cumulative sun exposure, especially on light colored skin, is one of the main risk factors, it is not surprising that with changing lifestyle and leisure activities, BCC can be diagnosed in younger adults more frequently. According to some publications, the life-time prevalence of each individual is already over 10% [3].
Compared to the high incidence of BCC, the mortality rate is fortunately very low as these tumors grow slowly and rarely metastasize. But depending on the tumor localization and size treatment can still be very challenging with a relevant number of recurrences and secondary tumors. The gold standard therapy for BCC, according to national guidelines, remains surgical excision with tumor-free margins, which is ideally achieved by microscopically controlled 3D-histology or Mohs surgery. Yet, the safety margin is a crucial issue. On one hand, it frankly is the aim to avoid recurrence, but on the other hand in head and neck surgery, it is also our goal to excise both as much as necessary and as little as possible as reconstruction becomes more complicated with the defect size [3-7].
Therefore, the focus of this study was to analyze the risk factors for relapse, to answer how much safety margin is necessary and whether incomplete resection automatically leads to a higher number of recurrences in BCC.
Material and Methods
All patients with a BCC of the head and neck who were treated in the Department of Oral and Maxillofacial Surgery of the Medical Center of the Johannes Gutenberg-University of Mainz between 2011 and 2014 were included in this study. Patients without valid histology or missing data were excluded. Epidemiological as well as therapeutic and histological data were collected from electronic health records. Tumor-specific data such as localization, maximum diameter, and histologic subtypes were noted.
Postoperative histologic reports were viewed concerning resection status (R0 vs. R+), and minimum safety margins (in mm, also based on the histological reports). Follow-up data had to be complete with at least one check-up once a year for at less five years. Otherwise, patients were excluded from our study. Follow-up records were explored for recurrences, which were defined as a histologically confirmed occurrence of a BCC in the same localization as the previous tumor.
Data were collected using SPSS 22.0, and statistical correlation was calculated with chi-squared-test, multivariate analysis and t-test for normally and Mann-Whitney-U-test for not normally distributed variables. The principles of the 1975 Declaration of Helsinki were followed in this study.
Results
Allover 141 patients (Table 1) could be included in the study with an average age of 72.9 years (±11.91; 64.3% ♂, 35.7% ♀). Most frequent tumor localization was the nose (24.1%), followed by the temple (9.9%), cheek (9.1%) and ear (8.5%). The mean tumor size was 22.8 mm (± 24.0 mm) with the biggest diameter of over 200mm. Nodular BCC was the most frequent histological subtype (45.4%), followed by sclerodermiform BCC (24.8%).
Table 1: Overview of the cases.
|
complete removal |
incomplete removal |
total |
|
|
n |
122 |
19 |
141 |
|
recurrence |
10 (8.2%) |
2 (10.5%) |
12 (8.5%) |
|
median diameter |
15.0 mm |
26.5 mm |
22.8 mm |
|
median age |
72.7 y |
74.6 y |
72.9 y |
|
gender |
61.5 % ♂, 38.5% ♀ |
84.2 % ♂, 15.8 % ♀ |
64.3% ♂, 35.7% ♀ |
|
number of tumors in the H-zone |
85 (69.7%) |
12 (63.2%) |
97 (68.8%) |
|
number of tumors in the M-zone |
37 (30.3%) |
7 (36.8%) |
44 (31.2%) |
|
sklerodermiformic BCC |
24.6 % |
26.3 % |
24.8% |
In all cases, microscopically controlled 3D-histology was performed with an investigation of the complete excisional border with the goal of a tumor-free excision and a histological safety margin of at least 3mm. However, only in 34% of the cases, a safety margin of more than 3mm was found in the histological reports. In 37.6% of the patients, total excision was achieved with a margin of less than 3mm, in 14.9% histological reports showed total excision but – mostly because of repeating excisions – it was not possible to reconstruct the exact safety margin. This means that in 86.5% of the cases, it was possible to successfully resect the tumor while in 13.5%, we found R+ status after at least one resection. In these patients, further excisions were not performed either because of patient denial, worsened general medical condition or because the surgeon agreed on the patient’s request on a watch-and-wait strategy. Overall, 8.5% of all patients showed a recurrence during the postoperative follow-up. The mean duration, until a recurrence was observed, was 15.6 months.
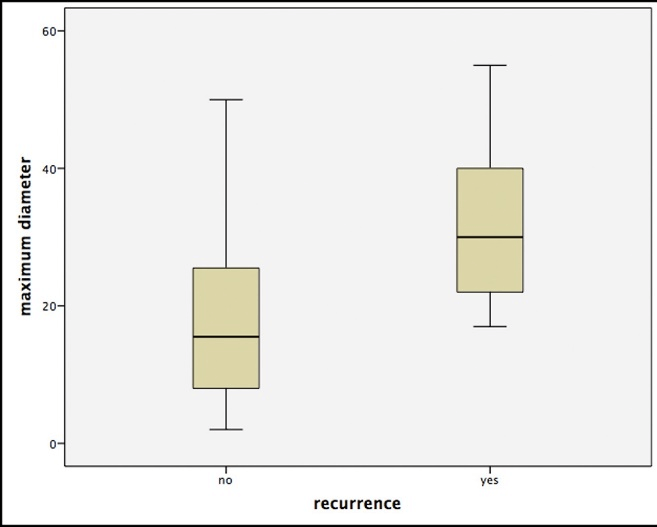
The comparison of the different histological subtypes showed significantly higher recurrence rates for sclerodermiformic BCC over any other entity in Chi-squared-test (p<0.05). Furthermore, we were able to identify the tumor diameter as an independent risk factor. This means that patients with recurrences had significantly larger primary BCCs (p<0.05, Figure 1). Additionally, multivariate analysis showed significant differences depending on the tumor localization, with the highest number of recurrences for the periorbital and nasal region (p<0.05).
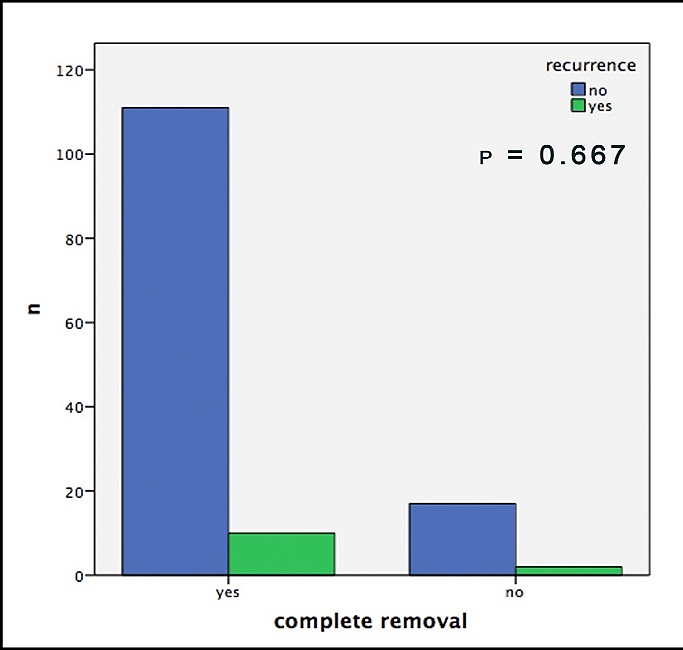
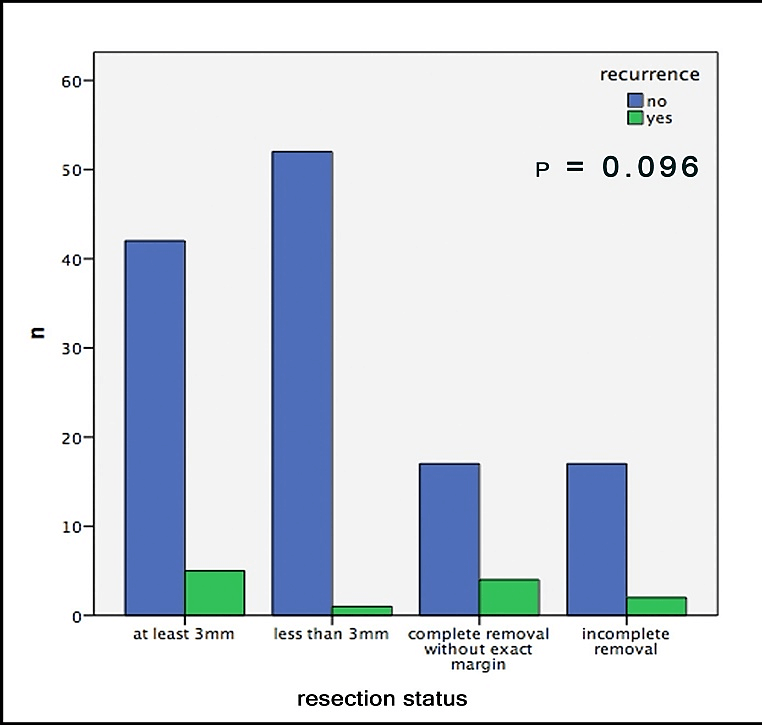
Out of 19 patients with incomplete surgical removal, 10.5% showed a recurrence during follow-up; out of those with complete removal this number was 8.2%. In this context, it is important to mention that both groups consisted of about the same amount of sclerodermiform/infiltrative BCCs, slightly more high-risk localizations in the R0 group and significantly larger tumors in the R+ group (Table1). Hence, we neither were able to find any significant correlation between recurrences and the resection-status (p=0.667, Figure 2). Furthermore, no significant difference could be found in the comparison of the safety margins (less than 3mm vs. more than 3mm) for completely removed BCCs (p=0.096, Figure 3).
Figure 1: Boxplots for the correlation of the maximum tumor size (in mm) and the occurrence of a relapse. Note that outliers were removed due to better illustration.
Figure 2: Histologically confirmed recurrences of the BCC depending on histological complete removal.
Figure 3: Recurrences of the BCC depending on margins.
Conclusion
The aim of this study was to investigate specific surgical, histological, and tumor-specific risk factors for the recurrence of BCCs of the head and neck. A retrospective assessment was conducted, and 141 patients who received surgical therapy for a BCC between 2011 and 2014 were included.
As described initially, in absolute contrast to its extremely high incidence of almost 200 cases per 100,000 inhabitants, BCC has very low mortality. This is mainly due to the slow growth of this tumor and the extremely low number of metastasis. Furthermore, in BCC, excellent tumor control can be achieved by surgical excision, which, based on several publications, reaches a cure rate of up to 99%. The overall recurrence rate after surgical excision is ranged between 2% and 8% and is the highest for tumors located in the face. In fact, the localization of a BCC has already been described as an independent risk factor for higher recurrence rates. More than two-thirds of the tumors of our study were located in the so-called H-zone, which has been published as a high-risk area for tumor recurrence. The H-zone comprises the nose, periorbital region, the lips, the area of the mandibular angle, the temples and periauricular skin. In our study, we observed a slightly higher number of relapses with 8.6%. In our opinion, this is mainly due to the high portion of large BCC and tumors located within the H-zone [1, 8-12].
In fact, the average size of tumors in our study was quite big compared to other publications, and our data show that with increasing tumor size, the risk for recurrences rises significantly. Similar to other entities, the tumor size plays an important role in the therapy of BCC, as already mentioned by Breuninger et al. According to the authors, the risk of remaining cancer cells due to subclinical tumor infiltration leading to incomplete resection correlates to the diameter of the primary. The updated German national guideline for the therapy of BCCs classifies different risk factors for tumor recurrence and states that depending on the localization, tumors of more than 6mm already involve higher numbers of recurrences [1, 13].
Another, yet well described factor for the recurrence of a BCC is the histological subtype. While nodular BCCs manifest the necessary resection margins more easily, sclerodermiformic BCCs are challenging because of their wide digitiform expansion and infiltration. In our study, almost 25% of all cases were sclerodermiformic, and we experienced a larger number of recurrences after surgical treatment of these tumors as well [1, 14, 15].
The most surprising result of this study, however, was that neither the safety margin nor the resection-status had an impact on the probability of a recurrence. This means that an incompletely removed BCC does not automatically lead to a relapse, which basically runs counter to logic. However, there is a number of publications that investigate incompletely excised BCCs by re-excision concerning residual tumor cells. These studies found the remaining cells in only about 50% of the cases. Accordingly, the number of recurrences in literature range between 26% and 41% [16-19].
Many different reasons can be found in this context. Some authors mention that after excision, coagulation of the margins for hemostasis might possibly kill remaining cells in the border of the tumor. However, this is a common surgical technique, which is performed in cases other than BCC, and therefore, if true, it ought to lead to the same effect in other entities as well. Another perhaps more plausible explanation is based on the regression of the remaining cancer cells. Tumor regression is an important characteristic which, for example, can be observed in malignant melanoma and is conducted by the invasion of CD4+ cells in the surrounding of a tumor. Regression has been shown for BCC, and few cases have been published that describe even spontaneous regression of untreated BCC. However, these cases are rare and mostly occur in immunocompromised transplant patients who discontinued medication due to organ rejection. The vast majority of studies that describe regression for BCC conclude that resection of the main tumor mass leads to antigen presentation to CD4+ cells that invade in the course of wound healing. These cells are then able to fulfill an immunological response, which leads to phagocytosis of the remaining tumor cells [7, 20-25].
Based on the results of this study, it is frankly not possible to give general advice on whether a BCC needs to be re-excised after incomplete removal. The authors also want to point out that in this study, the number of incomplete removals with recurrences was very low and that a retrospective study frankly has some drawbacks compared to prospective trials. However, the data still can show an important tendency. In our opinion, whenever total removal appears possible, this should be the absolute goal, especially, since surgical resection of recurrences are more challenging due to scarring, and as often, a more aggressive growth pattern can be observed in the relapse of a BCC [1, 16]. However, depending on the different factors such as histological type, localization and tumor size, it is possible to discuss a restrained attitude towards further excisions, for example, in older patients with a BCC in a low-risk localization.
Author Contributions
|
RK Rahimi-Nedjat |
Conception and design of the study Acquisition of data Interpretation of data Drafting of the article |
|
A Tuettenberg |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
|
C Loquai |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
|
K Sagheb |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
|
S Grabbe |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
|
S Hümmer |
Acquisition of data Interpretation of data Drafting of the article |
|
C Walter |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
|
B Al-Nawas |
Interpretation of data Revising of the manuscript Final approval of the manuscript |
Acknowledgement
We thank Kathy Taylor for providing language help.
Funding
None.
Conflicts of Interest
The authors declare to have given paid speeches, to have participated in advisory boards for pharmaceutical companies and to have conducted clinical approval trials on medication for the treatment of basal cell carcinoma. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Ethical Approval
Not applicable as there has been no work on human subjects in this study.
Abbreviations
BCC: basal cell carcinoma
NMSC: non-melanoma skin cancer
Article Info
Article Type
Research ArticlePublication history
Received: Fri 08, May 2020Accepted: Fri 22, May 2020
Published: Wed 17, Jun 2020
Copyright
© 2023 Roman Kia Rahimi-Nedjat. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.03.07
Author Info
Roman Kia Rahimi-Nedjat Andrea Tuettenberg Keyvan Sagheb Stephan Grabbe Carmen Loquai Sarah Hümmer Christian Walter Bilal Al-Nawas
Corresponding Author
Roman Kia Rahimi-NedjatDepartment of Oral and Maxillofacial Surgery - Plastic Surgery, University Medical Center, Johannes Gutenberg-University, Mainz, Germany
Figures & Tables
Table 1: Overview of the cases.
|
complete removal |
incomplete removal |
total |
|
|
n |
122 |
19 |
141 |
|
recurrence |
10 (8.2%) |
2 (10.5%) |
12 (8.5%) |
|
median diameter |
15.0 mm |
26.5 mm |
22.8 mm |
|
median age |
72.7 y |
74.6 y |
72.9 y |
|
gender |
61.5 % ♂, 38.5% ♀ |
84.2 % ♂, 15.8 % ♀ |
64.3% ♂, 35.7% ♀ |
|
number of tumors in the H-zone |
85 (69.7%) |
12 (63.2%) |
97 (68.8%) |
|
number of tumors in the M-zone |
37 (30.3%) |
7 (36.8%) |
44 (31.2%) |
|
sklerodermiformic BCC |
24.6 % |
26.3 % |
24.8% |
References
- AWMF (2018) S2K-Leitlinie Basalzellkarzinom.
- Chahal HS, Rieger KE, Sarin KY (2017) Incidence ratio of basal cell carcinoma to squamous cell carcinoma equalizes with age. J Am Acad Dermatol 76: 353-354. [Crossref]
- Berking C, Hauschild A, Kölbl O, Mast G, Gutzmer R (2014) Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch Arztebl Int 111: 389-395. [Crossref]
- Hauschild A, Breuninger H, Kaufmann R, Kortmann RD, Klein M et al. (2013) Brief S2k guidelines--Basal cell carcinoma of the skin. J Dtsch Dermatol Ges 11 Suppl 3: 10-16. [Crossref]
- Kovarik CL, Stewart D, Barnard JJ (2005) Lethal basal cell carcinoma secondary to cerebral invasion. J Am Acad Dermatol 52: 149-151. [Crossref]
- Lear JT, Smith AG (1997) Basal cell carcinoma. Postgrad Med J 73: 538-542. [Crossref]
- Stewart CM, Garlick J, Mcmullin J, Siddiqi F, Crombie C et al. (2015) Surgical Excision of Non-Melanoma Skin Cancer in an Elderly Veteran's Affairs Population. Plast Reconstr Surg Glob Open 2: e277. [Crossref]
- Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R et al. (2015) Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg 41: 550-571. [Crossref]
- Bath Hextall F, Bong J, Perkins W, Williams H (2004) Interventions for basal cell carcinoma of the skin: systematic review. BMJ 329: 705. [Crossref]
- Bath Hextall FJ, Perkins W, Bong J, Williams HC (2007) Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev 1: CD003412. [Crossref]
- Smeets NW, Krekels GA, Ostertag JU, Essers BA, Dirksen CD et al. (2004) Surgical excision vs Mohs' micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet 364: 1766-1772. [Crossref]
- Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS et al. (2016) Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 14: 574-597. [Crossref]
- Breuninger H, Dietz K (1991) Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol 17: 574-578. [Crossref]
- Husein ElAhmed H (2018) Sclerodermiform basal cell carcinoma: how much can we rely on dermatoscopy to differentiate from non-aggressive basal cell carcinomas? Analysis of 1256 cases. An Bras Dermatol 93: 229-232. [Crossref]
- Sartore L, Lancerotto L, Salmaso M, Giatsidis G, Paccagnella O et al. (2011) Facial basal cell carcinoma: analysis of recurrence and follow-up strategies. Oncol Rep 26: 1423-1429. [Crossref]
- Boulinguez S, Grison Tabone C, Lamant L, Valmary S, Viraben R et al. (2004) Histological evolution of recurrent basal cell carcinoma and therapeutic implications for incompletely excised lesions. Br J Dermatol 151: 623-626. [Crossref]
- Bozan A, Gode S, Kaya I, Yaman B, Uslu M et al. (2015) Long-term Follow-up of Positive Surgical Margins in Basal Cell Carcinoma of the Face. Dermatol Surg 41: 761-767. [Crossref]
- Masud D, Moustaki M, Staruch R, Dheansa B (2016) Basal cell carcinomata: Risk factors for incomplete excision and results of re-excision. J Plast Reconstr Aesthet Surg 69: 652-656. [Crossref]
- Robinson JK, Fisher SG (2000) Recurrent basal cell carcinoma after incomplete resection. Arch Dermatol 136: 1318-1324. [Crossref]
- Fujimura T, Kakizaki A, Kambayashi Y, Aiba S (2012) Basal cell carcinoma with spontaneous regression: a case report and immunohistochemical study. Case Rep Dermatol 4: 125-132. [Crossref]
- Hunt MJ, Halliday GM, Weedon D, Cooke BE, Barnetson RS (1994) Regression in basal cell carcinoma: an immunohistochemical analysis. Br J Dermatol 130: 1-8. [Crossref]
- Lespi PJ, Gregorini SD (2000) Folliculotropic T cells in regressive basal cell carcinoma of skin. Am J Dermatopathol 22: 30-33. [Crossref]
- Rawlins J, Platt A, Gowda P (2006) Regression of BCC following immunosuppression withdrawal in a renal transplant recipient. Clin Exp Dermatol 31: 717-718. [Crossref]
- Swetter SM, Boldrick JC, Pierre P, Wong P, Egbert BM (2003) Effects of biopsy-induced wound healing on residual basal cell and squamous cell carcinomas: rate of tumor regression in excisional specimens. J Cutan Pathol 30: 139-146. [Crossref]
- Yuan Y, Duff ML, Sammons DL, Wu S (2014) Retrospective chart review of skin cancer presence in the wide excisions. World J Clin Cases 2: 52-56. [Crossref]
