Response Time in Somatosensory Discrimination Tasks is Sensitive to Neurological Insult
A B S T R A C T
Background: A number of reports have demonstrated significant differences in human performance on diverse somatosensory-based discriminatory tasks dependent on the individual's neurological status. For example, compromised neurological status has been shown to lead to poor performance on tactile-based tasks such as vibrotactile stimulus amplitude discrimination, frequency discrimination, temporal order judgement, timing perception, and reaction time, and these deficits have been observed across a diverse spectrum of neurological disorders.
Results: In this report, response time of recently concussed individuals (1-3 days) was found to be significantly longer (~25%) than that of non-concussed individuals (i.e., controls) and individuals recovering from concussion (10+ days post-concussion). Additionally, a significant difference was found in response time on two different tasks. Timing perception, which is hypothesized to engage significantly more neural circuitry than amplitude discrimination, had a significantly longer average response time than amplitude discrimination.
Conclusions: These findings strongly suggest that response time could be used as a discriminative measure when evaluating overall neurological health and/or cognitive function, and this is consistent with findings of other reports that examined speed-accuracy trade-offs on discrimination tasks.
Keywords
Somatosensory, response time, discrimination task, amplitude discrimination, duration discrimination
Background
Over the past decade, we have been developing tactile-based neurosensory assessment tasks to utilize in evaluating CNS deficits across a wide spectrum of neurological disorders. The somatosensory-based tests have demonstrated sensitivity to alterations in neurological function in autism, Tourette's, OCD, ADHD, Parkinson's, chronic pain, concussion, aging, alcohol consumption history, early stage diabetes, and amputation [1-22]. Additionally, healthy individuals demonstrated sensitivity to pharmacological manipulation: low dose of DXM, conditioning with TMS and different conditions of adaptation [23-28]. In all of these studies, the primary observations were made by evaluating subject performance on multiple types of two-forced-choice alternative modified von Bekesy tracking protocol (first described in Tannan et al., 2005) [29]. In each of these protocols, the individual was queried to select the locus of one of two stimuli (e.g., which of the two stimuli was larger, which of the two stimuli was presented first, or which of the two stimuli was perceived to be longer in duration) and a tracking protocol adjusted the difference between the two stimuli presented on the subsequent trial based on subject's response (see Puts for comprehensive description of a full battery of tasks) [30]. The only value reported in the aforementioned reports was the difference limen obtained from the tracking protocols. An additional measure that is routinely collected with these two-forced-choice protocols, but has yet to be reported, is the time after a stimulus elapses before an individual respond with the answer (i.e., the "response time"). Response time, as it is defined in this report, is significantly different from the commonly used reaction time, a measure obtained by instructing an individual to respond as quickly as possible after they detect a stimulus. Rather, the response time is a measure that has been utilized in evaluating speed-accuracy trade-offs (SAT; see review by Heitz) in a wide range of discrimination tasks [31]. However, there have been no evaluations of the SAT in tactile-based discrimination tasks. Our primary objective in evaluating the response time was to determine if additional information about a subject's neurological condition could be obtained from discrimination tasks that are routinely administered in a wide range of studies.
There were two working hypotheses that guided this investigation. The first was that individuals with some type of neurological insult would have slower than normal response times, and the second was that timing perception - a task that theoretically would be more demanding than amplitude discrimination - would have a longer response time than amplitude discrimination. In this study, we investigated the response times of individuals with concussion and compared them with healthy controls. Although individuals were never instructed to respond quickly - only accurately - there appears to be a significant difference between the response time of healthy controls, concussed individuals, and individuals recovering from concussion. Additionally, the response time on tasks that are cognitively more demanding is longer for both healthy controls and concussed individuals. To date, there have been no reports of response time on tactile-based neurosensory assessments.
Methods
Data were collected from 102 healthy subjects (58 males, 44 females, mean age 19.6 years, SD 0.75 years) and 121 subjects (90 males, 31female, mean age 20.4, SD 1.49 years) who had sports-related concussions. All athletes were diagnosed with mild traumatic brain injury (mTBI) in the form of a concussion by a certified athletic trainer and the team physician with the help of the Sport Concussion Assessment Tool 2 (SCAT-2) and had no prior history of concussion or any other diagnosed medical conditions. A survey about medication and medical history was filled out by each subject before experimental tests to exclude subjects with a history of neurological impairment. All participating subjects were nadve of the study design and issue under investigation. The study was performed in accordance with the Declaration of Helsinki, all subjects gave their written informed consent, and the experimental procedures were reviewed and approved in advance by an institutional review board. A Brain Gauge stimulator (Figure 1) was used to deliver vibrotactile stimulation to the subjects during the experiments. The Brain Gauge vibrotactile stimulator was developed in our laboratories for use in experiments such as those described in this report. The design was based on the functionally equivalent CM4. The CM4 stimulator, described in detail in Holden et al., 2012, has been utilized to assess multiple sensory information processing characteristics in a diverse spectrum of human subject study [15, 20, 26, 28, 32-43]. The prominent feature of these protocols, which have demonstrated significant sensitivity to alterations in CNS processing, is that they are independent of detection thresholds or skin sensitivity.
During the evaluation session, subjects were seated comfortably in a chair with their hand on the Brain Gauge. Vibrotactile stimulation was delivered via 5 mm diameter probes that come in contact with the subject's digit 2 (D2; index finger) and digit 3 (D3; middle finger) of the left hand. The independent probe tips are computer-controlled and capable of delivering a wide range of sinusoidal vibrotactile stimulations of varying frequencies and amplitudes. The fingertip pads were chosen as test sites for two reasons: (1) to allow the convenience of access and comfort of the subject; and (2) because of the wealth of neurophysiological information that exists for the corresponding somatotopic regions of the cortex in primates. The subject used his/her right hand to indicate responses on a two-button computer mouse. A computer monitor provided visual cueing during each of the experimental runs. The cues indicated when the experimental stimuli would be delivered and when subjects were to respond. Training trials conducted prior to each task familiarized subjects with the test; correct responses on three consecutive training trials were required before the start of each assessment. The subject was given performance feedback during the training trials (the results of which were excluded from the collected data) but was not given performance feedback or knowledge of the results during data acquisition.
A series of sensory perceptual measures were employed to assess tactile information processing ability. Stimulus parameters were specified interactively by test algorithms based on specific protocols and the responses of the subjects during those protocols. In sum, these tests lasted approximately ten minutes and consisted of evaluations of amplitude discrimination and duration discrimination. Subjects were tested no more than six total times over the course of 1-2 weeks, with the exact number of testing sessions varying from subject to subject based on a number of factors. Researchers were instructed to test concussed subjects within 24 hours of the injury, at 48 hours, 4 days, 7 days and 10 days, as suitable for both the participant and the researcher's schedule. Variation in the testing intervals were due to the severity of the subject's injury, scheduling conflicts between the researcher and the participant, and/or the subject's decision to discontinue the study due to progress made towards recovery.
Figure 1: Brain Gauge two-point vibrotactile stimulator used in cortical metrics studies. Vertical skin displacement sinusoidal stimuli are delivered to the tips of the index and middle fingers via two round 5 mm diameter probes; the device interfaces to any computer or laptop via USB.
I Amplitude Discrimination (AD)
Amplitude discriminative capacity is defined as the minimal difference in amplitudes of two sinusoidal vibratory stimuli for which an individual can successfully identify the stimulus of larger magnitude. For the amplitude discrimination task, the device sequentially delivered sinusoidal vibrotactile stimuli (initial stimulus parameters: 400 µm peak-to-peak amplitude "test" stimulus, 200 µm "standard" stimulus, 25 Hz, 500 msec, 20 µm step size) to D2 and D3 over 20 trials. Inter-stimulus interval between the two stimuli was 500 msec. Discrimination capacity was assessed using a 2AFC tracking protocol that has been described and implemented in a number of previous studies [3, 23, 33, 37, 41-44]. The loci of the stimuli were randomly varied on a trial-by-trial basis, and subjects were questioned as to which of the two digits received the higher magnitude stimulus. The amplitude of the test stimulus was adjusted after each trial on the basis of the response such that correct responses lowered, while incorrect responses increased, the test amplitude on subsequent trials.
II Duration Discrimination (DD)
Duration discriminative capacity is defined as the minimal difference in durations of two stimuli for which an individual can successfully identify the stimulus of longer duration. For the duration discrimination task, sequential stimuli were delivered to D2 and D3 in 20 trials (initial stimulus parameters: 750 msec "test" stimulus, 500 msec "standard" stimulus, 300 µm, 40 Hz, 25 msec step size). Discrimination capacity was assessed using a 2AFC tracking protocol, and the location of the stimulus of longer duration was randomly selected on a trial-by-trial basis. Subjects were asked to indicate which of the two digits received the longer stimulus duration and, as previously reported subsequent duration of the test stimulus was adjusted on the basis of subject response [33].
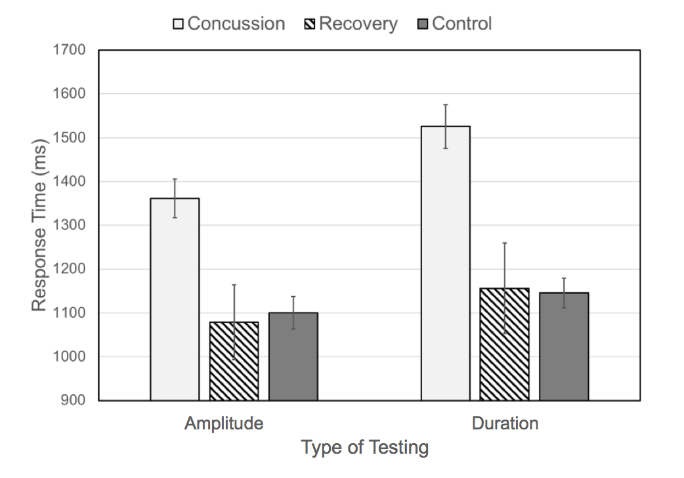
Figure 2: Average response time for amplitude discrimination and duration discrimination for control subjects, concussion subjects, and recovery subjects. Note that average response times for concussed individuals are significantly higher than those for controls and individuals recovering from concussion (error bars are standard error of the mean).
Results
The average response times obtained during the amplitude and duration discrimination tasks for healthy controls, recently concussed individuals, and individuals recovering from concussion are summarized in Figure 2. The Concussion group includes data points collected 0 to 3 days after a subject was diagnosed with concussion and the Recovery group includes data collected at least 10 days after sustaining a concussion. The response times collected from the concussion group are significantly longer than those collected from the healthy controls or the subjects after recovery. The average response time on the amplitude discrimination task was 1353 ± 44 msec for the concussion group, approximately 23% higher than the average response time for healthy controls (1100 ± 37 msec) and the recovery group (1079 ± 85 msec). Similarly, the average response time on the duration discrimination task was 1520 ± 49 msec for the concussion group, approximately 32% higher than the average response time for healthy controls (1146 ± 34 msec) and the recovery group (1156 ± 103 msec). It is worth noting the similarity between the average response times for controls and subjects in the recovery group not only between groups (21 msec for amplitude discrimination; 10 msec for duration discrimination) but also between tasks (<80 msec between all average response times for both groups). In contrast, the average response time for subjects in the concussion group was 167 msec higher on the duration discrimination task compared to the amplitude discrimination task.
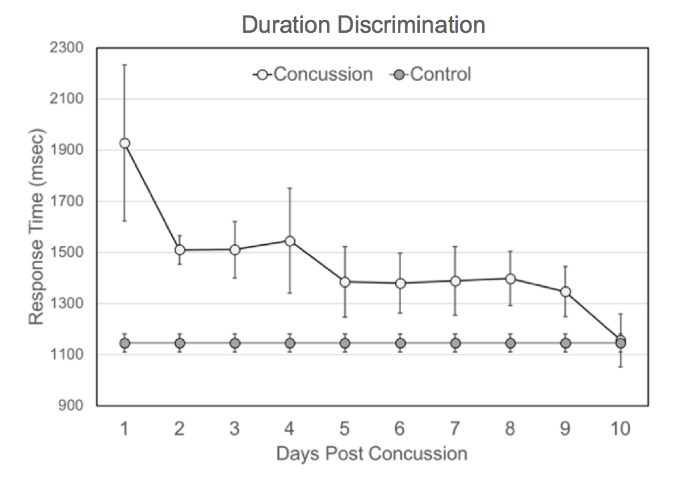
Figure 3 shows how the average response time in the duration discrimination task changes as subjects diagnosed with a concussion recover. It is clear from the graph that within the first 24-72 hours, subjects have much higher response times in the duration discrimination task, with statistically elevated response times remaining until 10 days after injury (Single Factor ANOVA analysis shows a significant difference between healthy controls and subjects 9 days after concussion [F(1,119)=4.87 p=0.029]).
Figure 3: Average response time on duration discrimination task for concussion subjects monitored for 10 days post-concussion. Concussed individuals have much higher response times immediately following concussion (within 24 hours) compared to controls (~800 msec longer response time). The average response time remains elevated for up to 9 days before recovering to the control range. The control average was plotted on each day for the purpose of comparison to the concussion group and was not measured each of the 10 days shown.
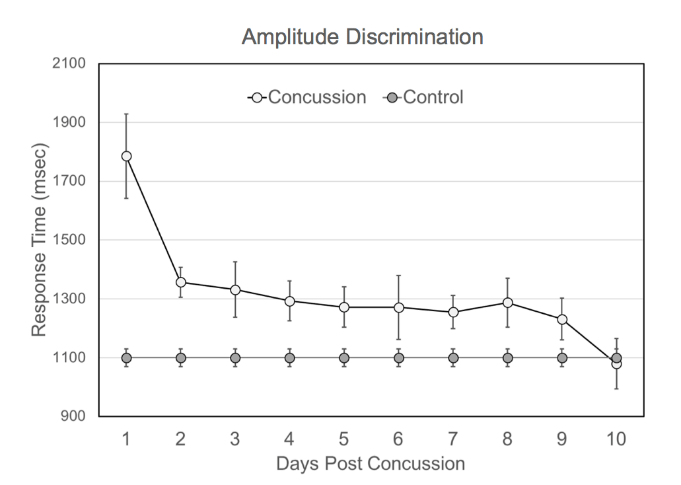
Figure 4 shows how the average response time in the amplitude discrimination task changes as subjects diagnosed with a concussion recover. It is clear from the graph that within the first 24-72 hours, subjects have much higher response times in the amplitude discrimination task, with statistically elevated response times remaining until 9 days after injury (Single Factor ANOVA analysis shows a significant difference between healthy controls and subjects 8 days after concussion [F(1,125)=4.75 p=0.032]).
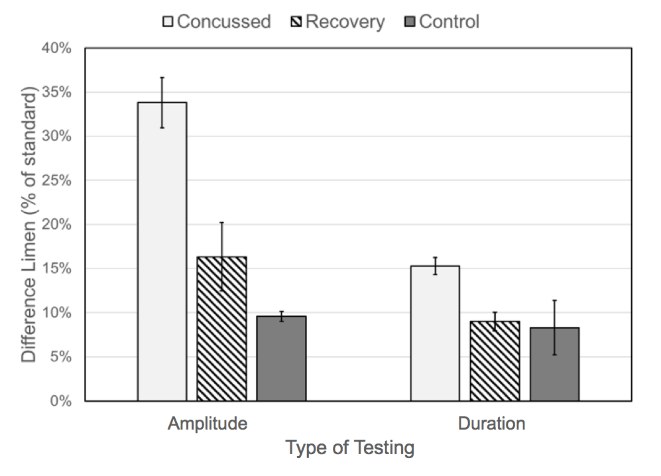
Subject performance in terms of average difference limen (DL) for healthy controls and the concussion and recovery groups are plotted in (Figure 5). The difference limens were all normalized to the standard used for testing; amplitude discrimination DLs were divided by 400 ?m and duration discrimination DLs were divided by 500 msec. The DLs collected from the concussion group are significantly larger than those collected from the healthy controls or the subjects after recovery. The average DL in the amplitude discrimination task was 34±3% for the concussion group, higher than the average DL for healthy controls (10±1%) and the recovery group (16±4%). Similarly, the average DL for the duration discrimination task was 15±1% for the concussion group, marginally higher than the average DL for healthy controls (8±3%) and the recovery group (9±1%).
In order to better understand the relationship between performance and response time, Pearson's correlation coefficients were calculated for each test. The control group showed very little causal relationship between response time and the duration discrimination DL (r=0.198) or response time and the amplitude discrimination DL (r=0.04). A very weak relationship was observed for the concussed individuals between response time and both duration discrimination DL (r=0.239) and amplitude discrimination DL(r=0.249).
Figure 4: Average response time to amplitude discrimination task for concussion subjects monitored for 10 days post-concussion. Concussed individuals have much higher response times immediately following concussion (within 24 hours) compared to controls (~700 msec longer response time). The average response time remains elevated for up to 8 days before recovering to the control range. The control average was plotted on each day for the purpose of comparison to the concussion group and was not measured each of the 10 days shown.
Figure 5: Difference limen of amplitude discrimination and duration discrimination (shown as a percentage normalized to the standard) for control subjects, concussion subjects and recovery subjects.
Discussion
There are two significant findings in this study. The first finding is that the concussed individuals were much slower on task performance for both the amplitude discrimination task and the timing perception task than they were post-recovery. Additionally, the response time of concussed subjects was slower than healthy controls and it appears that concussed individuals are simply not as efficient at either of the tasks described. The second finding is that although duration discrimination task response time was only moderately slower for healthy controls, it was significantly higher for concussed individuals. The difference in the response time for the two tasks - amplitude discrimination and duration discrimination - is most likely due to the neural pathways that are engaged to accomplish one task versus the other. There is no doubt that in both tasks the parietal cortex is involved in processing stimuli, and both tasks undoubtedly engage the frontal lobes for decision making. The increase in response time in the timing perception task is most likely due to the engagement of the cerebellum. The ability of an individual to differentiate which of two stimulus epochs is longer in duration has been attributed to cerebellar-cortical circuitry: in studies in which TMS was used to block activity in the cerebellum, timing perception task performance was greatly reduced or eliminated regardless of which sensory modality was used [45]. The interesting finding of note is that the difference in response times for the two tasks increased significantly for the concussed individuals, and the authors suspect that this is indicative of increased delays in information processing that are exaggerated by an expansion in the cortical circuitry engaged by the slightly more complex task.
An obvious future investigation that this finding suggests is a study in which subjects are instructed to respond as quickly as possible while trying to answer correctly. The authors suspect that accuracy on the tasks would be compromised, but this will be an area of future investigation. We regard this speed-accuracy trade-off (SAT) as an indication of efficiency: the longer it takes an individual to respond to the task, the less efficient their task performance. The speed-accuracy trade-off on discrimination tasks has been studied and documented extensively since the late 19th century, with the ubiquitous and unsurprising finding that a focus on speed decreases accuracy of the task [31]. More recent studies have shifted focus towards the use of tools such as fMRI and EEG as well as deviations from the normal response in individuals with neurocognitive disorders or drug use in order to better understand the processes taking place [46-64]. fMRI studies have shown an increase in activity in the striatum and pre-supplementary motor area when speed is emphasized for a task [48, 49, 51, 53, 54]. Evidence points towards increased baseline activity in pre-motor areas, but not in sensory cortical or primary motor areas [47]. EEG studies confirm the finding that speed emphasis does not affect sensory processing, and if that is the case, then accuracy on the discrimination tasks would not be compromised by the reduced response time [58, 59, 63].
An interesting finding of this study appears to be that the DL for amplitude discrimination deviates more from control values for concussed individuals than does the DL for duration discrimination, yet the reverse appears to hold true for response time. For response time, the duration discrimination value deviated more from control values for the concussed condition. This leads to some interesting questions about the complexity of sensory perceptual tasks to be left for future studies. It is not completely predictable how accuracy might change with different instructions. In this study, there was a very weak, or lack of, correlation between accuracy and response time. Instructing individuals to respond more rapidly could have a significant impact on the accuracy score, particularly when comparing healthy controls to concussed individuals. For example, one of the few studies of SAT in individuals with neurocognitive disorders focuses on adolescents with ADHD. Mulder et al. found that when adolescents with ADHD were instructed to respond accurately to a visual task, they responded more quickly than and with equal accuracy of healthy age-matched controls on a visual task [65]. However, when subjects were told to respond as quickly as possible, the adolescents with ADHD had similar speed and accuracy to healthy controls. The use of drugs has also been investigated in relation to SAT processing. Alcohol, in particular, has been shown to decrease accuracy on a range of tests, but changes in speed relative to a placebo control group are variable [66-69]. In contrast, the dopamine agonist bromocriptine showed no change in SAT compared to placebo control [70]. Thus, it appears that neurological alterations have an unpredictable influence on the speed-accuracy trade off.
The difference observed in this study for response time between concussed and non-concussed individuals suggests that regardless of which tactile discriminative task is performed, the response time data could provide additional information that will strengthen the overall evaluation of an individual with neurological insult. Additionally, the difference between response times on the amplitude discrimination task versus the timing perception task increases significantly when a subject is concussed. One implication of this finding is that the response time difference is exaggerated by the neurological insult introduced by the concussion simply because of the additional cerebellar circuitry involved. In other words, decreasing overall information processing speed of the CNS will result in a much longer response time for tasks involving significantly more circuitry. This hypothesis is supported by the current data, and future research will address this interesting question. The operational significance of this finding is that evaluating response time differences between two tasks obtained post neurological insult would not require a pre-injury measure to be obtained to determine an individual's status because the important measure could be the response time performance difference. Many different methods are currently being deployed for concussion assessments, the majority of which require pre-injury baseline assessments. Additional measures, such as the response time for discrimination task, could potentially improve overall assessment of individuals suspected of concussion, particularly when a multi-parametric approach is used [16].
Conclusion
The overall objective of our work is to develop metrics that are sensitive to alterations in neurological function, and although the response time metric is a by-product of other assessments, it appears that it could be very useful in evaluating a number of pathologies. Subsequent reports will describe response times for somatosensory discrimination tasks in other pathologies as well as a more comprehensive review of the differences in the neurosensory assessments observed between healthy controls and concussed individuals.
Ethics Approval and Consent to Participate
The research was performed in accordance with the Declaration of Helsinki, subjects gave their written consent, and the experiment design was reviewed by an Institutional Review Board.
Consent for Publication
Not Applicable.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
MT is co-founder of Cortical Metrics, which has a license from the University of North Carolina to distribute the Brain Gauge, the device used to conduct the study.
Funding
This work was supported, in part, by the Office of Naval Research (N00014-14-C-0378).
Author's Contributions
MT developed the underlying hypotheses and participated in the drafting of the manuscript. JH participated in the design and the conduct of the experiments. EF and RL participated in the statistical analysis of the collected data and the drafting of the manuscript. AT conducted the pilot data analysis, literature search, and drafted portions of the Background and Discussion.
Abbreviations
AD = Amplitude Discrimination
ADHD = Attention Deficit Hyperactive Disorder
CNS = Central Nervous System
D2 = Digit Two
D3 = Digit Three
DD = Duration Discrimination
DL = Difference Limen
DXM = Dextromethorphan
mTBI = mild Traumatic Brain Injury
msec = milliseconds
OCD = Obsessive Compulsive Disorder
SAT = Speed-Accuracy Trade-Offs
TMS = Transcranial Magnetic Stimulation
Article Info
Article Type
Research ArticlePublication history
Received: Fri 25, Oct 2019Accepted: Thu 28, Nov 2019
Published: Wed 11, Dec 2019
Copyright
© 2023 Tommerdahl M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2019.04.01
Author Info
Favorov OV Francisco EM Holden JK Lensch R Tommerdahl AP Tommerdahl M
Corresponding Author
Tommerdahl MCortical Metrics, LLC
Figures & Tables

References
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL (2007) Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116-123. [Crossref]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT (2008) Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local underconnectivity? Behav Brain Funct BBF 4: 19.
- Tannan V, Holden JK, Zhang Z, Baranek GT, Tommerdahl MA (2008) Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res Off J Int Soc Autism Res 1: 223-230. [Crossref]
- Puts NAJ, Wodka E, Tommerdahl M, Barker P, Mostofsky S, Edden RAE (2013) A Combined GABA-MRS and Behavioral Study in Children with Autism Spectrum Disorder. International Meeting for Autism Research
- Puts NAJ, Wodka EL, Tommerdahl M, Mostofsky S, Edden R (2014) Impaired Tactile Processing in Children with Autism Spectrum Disorder. J Neurophysiol 111: 1803-1811. [Crossref]
- Tavassoli T, Belleshem K, Tommerdahl M, Holden JK, Grodberg D et al. (2013) Tactile Reactivity in Children with and without Autism Spectrum Disorders. in: International Meeting for Autism Research. Presented at the International Meeting for Autism Research, Madrid.
- Francisco E, Favorov O, Tommerdahl M (2013) The Role of Cortical Modularity in Tactile Information Processing: An Approach to Measuring Information Processing Deficits in Autism. Recent Advances in Autism Spectrum Disorders - Volume II InTech
- Puts NAJ, Harris AD, Crocetti D, Nettles C, Singer HS et al. (2015) Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol 114: 808-817. [Crossref]
- Güçlü B, Tanıdır C, Çanayaz E, Güner B, İpek Toz H et al. (2015) Tactile processing in children and adolescents with obsessive-compulsive disorder. Somatosens Mot Res 32: 163-171. [Crossref]
- Puts NAJ, Harris AD, Mikkelsen M, Tommerdahl M, Edden RAE et al. (2017) Altered tactile sensitivity in children with attention-deficit hyperactivity disorder. J Neurophysiol 118: 2568-2578. [Crossref]
- Nelson AJ, Premji A, Rai N, Hoque T, Tommerdahl M, Chen R (2012) Dopamine alters tactile perception in Parkinson’s disease. Can J Neurol Sci 39: 52-57. [Crossref]
- Kursun O, Sakar B, Isenkul M, Sakar C, Gurgen F et al. (2013) Analysis of Effects of Parkinson’s Disease on the Somatosensory System via CM-4 Tactile Stimulator.
- Zhang Z, Zolnoun DA, Francisco EM, Holden JK, Dennis RG et al. (2011) Altered central sensitization in subgroups of women with vulvodynia. Clin J Pain 27: 755-763. [Crossref]
- Maeda Y, Kettner N, Holden J, Lee J, Kim J et al. (2014) Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain 137: 1741-1752. [Crossref]
- Nguyen R, Ford S, Calhoun AH, Holden J, Gracely RH et al. (2013) Neurosensory assessments of migraine. Brain Res 1498: 50-58. [Crossref]
- Tommerdahl M, Dennis RG, Francisco EM, Holden JK, Nguyen R et al. (2016) Neurosensory assessments of concussion. Mil Med 181: 45-50. [Crossref]
- King HE (1962) Reaction-Time as a Function of Stimulus Intensity Among Normal and Psychotic Subjects. J Psychol 54: 299-307.
- Favorov O, Francisco E, Zai L, Tommerdahl M (2017) Quantification of Mild Traumatic Brain Injury Via Cortical Metrics: Analytical Methods.
- Zhang Z, Francisco EM, Holden JK, Dennis RG, Tommerdahl M (2011) Somatosensory Information Processing in the Aging Population. Front Aging Neurosci 3: 18. [Crossref]
- Nguyen RH, Gillen C, Garbutt JC, Kampov Polevoi A, Holden JK et al. (2013) Centrally mediated sensory information processing is impacted with increased alcohol consumption in college-aged individuals. Brain Res 1492: 53-62. [Crossref]
- Favorov OV, Kursun O, Tommerdahl M (2017) Role of Feed-Forward Inhibition in Neocortical Information Processing: Implications for Neurological Disorders, in: The Physics of the Mind and Brain Disorders, Springer Series in Cognitive and Neural Systems. Springer Cham pp: 383-397.
- Collins KL, McKean DL, Huff K, Tommerdahl M, Favorov OV et al. (2017) Hand-to-Face Remapping But No Differences in Temporal Discrimination Observed on the Intact Hand Following Unilateral Upper Limb Amputation. Front Neurol 8: 8. [Crossref]
- Folger SE, Tannan V, Zhang Z, Holden JK, Tommerdahl M (2008) Effects of the N-methyl-D-Aspartate receptor antagonist dextromethorphan on vibrotactile adaptation. BMC Neurosci 9: 87. [Crossref]
- Lee K, Jacobs M, Asmussen M, Zapallow C, Tommerdahl M et al. (2012) The Influence of Continuous Theta-Burst Stimulation over the Primary Somatosensory Cortex on Temporal Order Judgement Perception. Society for Neuroscience 377:12.
- Lee KG, Jacobs MF, Asmussen MJ, Zapallow CM, Tommerdahl M et al. (2013) Continuous theta-burst stimulation modulates tactile synchronization. BMC Neurosci 14: 89. [Crossref]
- Rai N, Premji A, Tommerdahl M, Nelson AJ (2012) Continuous theta-burst rTMS over primary somatosensory cortex modulates tactile perception on the hand. Clin Neurophysiol 123: 1226-1233. [Crossref]
- Jones CB, Lulic T, Bailey AZ, Mackenzie TN, Mi YQ et al. (2016) Metaplasticity in human primary somatosensory cortex: effects on physiology and tactile perception. J Neurophysiol 115: 2681-2691. [Crossref]
- Tannan V, Simons S, Dennis RG, Tommerdahl M (2007) Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res 1186: 164-170. [Crossref]
- Tannan V, Dennis R, Tommerdahl M (2005) A novel device for delivering two-site vibrotactile stimuli to the skin. J Neurosci Methods 147: 75-81. [Crossref]
- Puts NAJ, Edden RAE, Wodka EL, Mostofsky SH, Tommerdahl M (2013) A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J Neurosci Methods 218: 39-47. [Crossref]
- Heitz RP (2014) The speed-accuracy tradeoff: history, physiology, methodology, and behavior. Front Neurosci 8: 150. [Crossref]
- Holden J, Nguyen R, Francisco E, Zhang Z, Dennis RG et al. (2012) A novel device for the study of somatosensory information processing. J Neurosci Methods 204: 215-220. [Crossref]
- Francisco E, Tannan V, Zhang Z, Holden J, Tommerdahl M (2008) Vibrotactile amplitude discrimination capacity parallels magnitude changes in somatosensory cortex and follows Weber’s Law. Exp Brain Res 191: 49-56. [Crossref]
- Francisco E, Holden J, Zhang Z, Favorov O, Tommerdahl M (2011) Rate dependency of vibrotactile stimulus modulation. Brain Res 1415: 76-83. [Crossref]
- Nelson AJ, Premji A, Rai N, Hoque T, Tommerdahl M (2012) Dopamine alters tactile perception in Parkinson’s disease. Can J Neurol Sci 39: 52-57. [Crossref]
- Tannan V, Dennis RG, Tommerdahl M (2005) Stimulus-dependent effects on tactile spatial acuity. Behav Brain Funct 1: 18 [Crossref]
- Tannan V, Dennis RG, Zhang Z, Tommerdahl M (2007) A portable tactile sensory diagnostic device. J Neurosci Methods 164: 131-138. [Crossref]
- Tannan V, Holden JK, Zhang Z, Baranek GT, Tommerdahl MA (2008) Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res 1: 223-230. [Crossref]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL (2007) Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116-123. [Crossref]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL (2007) Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116-123. [Crossref]
- Zhang Z, Francisco E, Holden J, Dennis R, Tommerdahl M (2009) The impact of non-noxious heat on tactile information processing. Brain Res 1302: 97-105. [Crossref]
- Zhang Z, Francisco E, Holden J, Dennis R, Tommerdahl M (2011) Somatosensory Information Processing in the Aging Population. Front Aging Neurosci 3: 18. [Crossref]
- Zhang Z, Zolnoun D, Francisco E, Holden J, Dennis R et al. (2011) Altered central sensitization in subgroups of women with vulvodynia. Clin J Pain 27: 755-763. [Crossref]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL (2007) Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116-123. [Crossref]
- Koch G, Oliveri M, Caltagirone C (2009) Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc B Biol Sci 364:1907-1918. [Crossref]
- Blumen HM, Gazes Y, Habeck C, Kumar A, Steffener J et al. (2011) Neural Networks Associated with the Speed-Accuracy Tradeoff: Evidence from the Response Signal Method. Behav Brain Res 224: 397-402. [Crossref]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S (2010) The neural basis of the speed-accuracy tradeoff. Trends Neurosci 33: 10-16. [Crossref]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY et al. (2008) Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci U S A 105: 17538-17542. [Crossref]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S et al. (2010) Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci 107: 15916-15920.
- Ho T, Brown S, van Maanen L, Forstmann BU, Wagenmakers EJ et al. (2012) The optimality of sensory processing during the speed-accuracy tradeoff. J Neurosci Off J Soc Neurosci 32: 7992-8003. [Crossref]
- Ivanoff J, Branning P, Marois R (2008) fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PloS One 3: e2635. [Crossref]
- Vallesi A, McIntosh AR, Crescentini C, Stuss DT (2012) fMRI investigation of speed-accuracy strategy switching. Hum Brain Mapp 33: 1677-1688. [Crossref]
- van Maanen L, Brown SD, Eichele T, Wagenmakers EJ, Ho T et al. (2011) Neural correlates of trial-to-trial fluctuations in response caution. J Neurosci 31: 17488-17495. [Crossref]
- van Veen V, Krug MK, Carter CS (2008) The neural and computational basis of controlled speed-accuracy tradeoff during task performance. J Cogn Neurosci 20: 1952-1965. [Crossref]
- Zhang J (2012) The Effects of Evidence Bounds on Decision-Making: Theoretical and Empirical Developments. Front Psychol 3: 263. [Crossref]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993) A Neural System for Error Detection and Compensation. Psychol Sci 4: 385-390.
- Heitz RP, Schall JD (2013) Neural chronometry and coherency across speed-accuracy demands reveal lack of homomorphism between computational and neural mechanisms of evidence accumulation. Philos Trans R Soc Lond B Biol Sci 368:20130071. [Crossref]
- Lubbe R, Jáskowsky P, Wauschkuhn B, Verleger R (2001) Influence of Time Pressure in a Simple Response Task, a Choice-by-Location Task, and the Simon Task. J Psychophysiol 15.
- Osman A, Lou L, Muller Gethmann H, Rinkenauer G, Mattes S et al. (2000) Mechanisms of speed-accuracy tradeoff: evidence from covert motor processes. Biol Psychol 51: 173-199. [Crossref]
- Pastötter B, Berchtold F, Bäuml K HT (2012) Oscillatory correlates of controlled speed-accuracy tradeoff in a response-conflict task. Hum Brain Mapp 33: 1834-1849. [Crossref]
- Pfefferbaum A, Ford J, Johnson R, Wenegrat B, Kopell BS (1983) Manipulation of P3 latency: speed vs. accuracy instructions. Electroencephalogr. Clin Neurophysiol 55: 188-197. [Crossref]
- Rinkenauer G, Osman A, Ulrich R, Muller Gethmann H, Mattes S (2004) On the locus of speed-accuracy trade-off in reaction time: inferences from the lateralized readiness potential. J Exp Psychol Gen 133: 261-282. [Crossref]
- Wenzlaff H, Bauer M, Maess B, Heekeren HR (2011) Neural Characterization of the Speed–Accuracy Tradeoff in a Perceptual Decision-Making Task. J Neurosci 31: 1254-1266. [Crossref]
- van Vugt MK, Simen P, Nystrom LE, Holmes P, Cohen JD (2012) EEG oscillations reveal neural correlates of evidence accumulation. Front Neurosci 6: 106. [Crossref]
- Mulder MJ, Bos D, Weusten JMH, van Belle J, van Dijk SC et al. (2010) Basic impairments in regulating the speed-accuracy tradeoff predict symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry 68: 1114-1119. [Crossref]
- Jennings JR, Wood CC, Lawrence BE (1976) Effects of graded doses of alcohol on speed-accuracy tradeoff in choice reaction time. Percept Psychophys 19: 85-91.
- Rundell OH, Williams HL (1979) Alcohol and speed-accuracy tradeoff. Hum Factors 21: 433-443. [Crossref]
- Tiplady B, Drummond GB, Cameron E, Gray E, Hendry J et al. (2001) Ethanol, errors, and the speed-accuracy trade-off. Pharmacol Biochem Behav 69: 635-641. [Crossref]
- van Ravenzwaaij D, Dutilh G, Wagenmakers EJ (2012) A diffusion model decomposition of the effects of alcohol on perceptual decision making. Psychopharmacology (Berl.) 219: 1017-1025. [Crossref]
- Winkel J, van Maanen L, Ratcliff R, van der Schaaf ME, van Schouwenburg MR et al. (2012) Bromocriptine Does Not Alter Speed–Accuracy Tradeoff. Front Neurosci 6: 126. [Crossref]
