Requiring Multi-Steps During Minimally Invasive Surgery to Achieve Optimal Proximal Resection Margin for Siewert-Types II and III Esophagogastric Junctional Cancer
A B S T R A C T
Background: Transection of the esophagus at a cancer-negative proximal surgical margin and alimentary tract reconstruction through the hiatus during minimally invasive surgery (MIS) may be complicated and difficult in some patients with Siewert type II or III esophagogastric junctional cancer (EGJC). In this study, we retrospectively determined requiring multi-steps during MIS for Siewert types II and III EGJC.
Study Design: Fifty-one consecutive patients with surgically treated Siewert type II or III EGJC were reviewed from July 2006 to October 2016. Five patients were excluded, and the remaining forty-six patients were divided into four groups based on the combination of laparoscopic and thoracoscopic surgical procedures performed, according to Siewert classification and TNM-staging: one-step surgery (n = 16), two-step surgery without novel transection of the esophagus (n = 8), two-step surgery with novel transection of the esophagus (n = 13), and three-step surgery (n = 9).
Results: The esophagus was transected successfully with a cancer-free proximal margin in all but one patient. However, only 16 patients (35 %) were treated successfully by laparoscopic surgery alone, and the remaining 30 patients needed one or more additional steps to complete the anastomosis after transection of the esophagus according to the extent of esophageal invasion of the tumor.
Conclusion: Multi-step procedures may be needed to achieve a cancer-negative proximal margin followed by alimentary reconstruction during MIS in patients with Siewert type II or III EGJC.
Keywords
Esophagogastric junctional cancer, siewert classification, minimally invasive surgery
Introduction
The number of patients with esophageal or gastric cancer treated by minimally invasive surgery (MIS) using thoracoscopy, laparoscopy, or both has been increasing [1-5]. However, MIS for esophagogastric junctional cancer (EGJC) has not been well documented, despite the increased frequency of adenocarcinoma of the esophagogastric junction [6-9]. Surgery for EGJC involves total or proximal gastrectomy with or without radical esophagectomy, according to the extent of tumor infiltration in the esophagus and the lymph node metastasis status [10-12]. However, the transhiatal procedure on the mediastinal side is very complex in some cases due to dissection of the lower mediastinal lymph nodes, the need for a cancer-negative surgical proximal margin of the esophagus, and reconstruction of the alimentary tract with a short remnant esophagus [13-17]. It has recently been proposed that the optimal cancer-negative proximal surgical margin for long-term survival in patients with EGJC is ≥ 2 cm or 3.8 cm of the esophagus. In cases with EGJC or gastric cancer with long esophageal invasion, MIS procedures involving two or more-steps may be required to ensure transection of the esophagus with a cancer-negative proximal surgical margin and safe reconstruction of an alimentary tract [7, 18, 19]. On the other hands, some patients with EGJC may be undergone initial thoracoscopy for mediastinal lymphadenectomy or transection of the esophagus to confirm the surgical strategy, before undergoing the abdominal procedure.
We have treated patients with EGJC in our institutes according to a uniform surgical strategy based on the Siewert classification and the TNM staging system. In this study, we retrospectively reviewed a consecutive series of patients with Siewert-type II or III EGJC who underwent MIS, with a focus on the complexity of surgical steps required to transect the esophagus with a cancer-negative proximal surgical margin and to reconstruct the alimentary tract after resection.
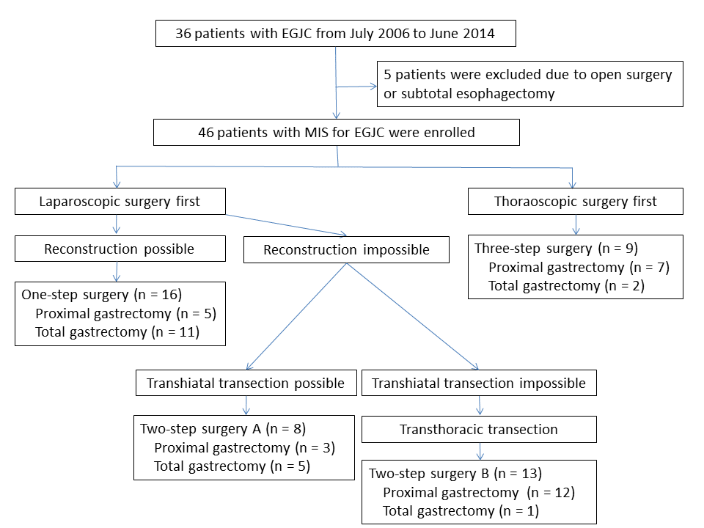
Figure 1: Consort diagram of patients enrolled in this study. One-step surgery: all surgical procedures performed under a laparoscope alone; two-step surgery: laparoscopic procedures followed by thoracoscopic procedures; three-step surgery: thoracoscopic procedures followed by laparoscopic procedures, followed by a second thoracoscopic procedure.
Patients and Methods
We started performing MIS for EGJC in July 2006 at our own or an affiliated hospital, since when patients with EGJC have generally undergone MIS. A total of 51 patients were treated surgically for a diagnosis of Siewert type II or III EGJC with curative intent from July 2006 until October 2017 [7]. Of these, three patients received combined thoracoscopic and open laparotomic procedures due to previous major laparotomic surgery, and two patients with advanced Siewert type II or III tumors underwent subtotal esophagectomy and cervical anastomosis. The remaining 46 patients who underwent totally MIS were enrolled in this study (Figure 1). Distant metastasis, multi-organ involvement, enlarged cervical lymph nodes, and findings suggestive of para-aortic lymph node metastasis on computed tomography or fluorodeoxyglucose-positron emission tomography scans were considered to be indications of incurable disease.
I Surgical Strategy
Our surgical strategy for treating EGJC based on the Siewert classification for adenocarcinoma of the esophagogastric junction was reported in 2012 [7, 20]. This was largely compatible with the surgical therapeutic strategy based on the results of a nation-wide retrospective study of lymphadenectomy for EGJC [21]. One-step transhiatal laparoscopic surgery was the initial choice in patients with Siewert type II or III EGJC. Otherwise, patients scheduled for MIS underwent multiple surgical steps involving a combination of transthoracic and transhiatal procedures. Early stage Siewert-type II or III tumors were treated by a transhiatal abdominal approach, including cardiectomy with dissection of the proximal perigastric lymph nodes, the nodes along the left gastric artery and the lower mediastinal lymph nodes through an abdominal approach, as well as esophagogastrostomy. Advanced EGJC of type II or III invading < 3 cm into the esophagus were treated by a transhiatal abdominal approach including a proximal or total gastrectomy with dissection of the perigastric lymph nodes, regional second tier-nodes along the left gastric, common hepatic, splenic and celiac arteries and lower mediastinal lymph nodes, and esophagogastrostomy or Roux-en-Y esophagojejunostomy. Patients with advanced type II or III cancer invading > 3 cm into the esophagus were treated using a transthoracic and abdominal approach for adequate lower mediastinal lymph node dissection and to obtain a safe surgical margin if subtotal esophagectomy with middle-upper mediastinal lymph node dissection was avoidable. If the gastric remnant after proximal gastrectomy was relatively small, jejunal interposition reconstruction was selected to prevent reflux esophagitis. Treatments in other patients, such as those with a sliding hiatal hernia, were planed individually according to the proximal and distal extensions of the tumor and the possible lymph node metastasis status. The proximal transection line at the esophagus was predesigned by preoperative upper gastrointestinal series or endoscopy or confirmed by marking clips placed preoperatively or by intraoperative endoscopic examination. Patients were divided into four groups based on the combination of laparoscopic and thoracoscopic surgical procedures performed, according to Siewert classification and TNM-staging: one-step surgery, two-step surgery without novel transection of the esophagus, two-step surgery with novel transection of the esophagus, and three-step surgery.
II Laparoscopic Procedure Alone Group (One-Step Surgery Group)
Patients in the one-step surgery group were treated by one-step laparoscopic surgery with total or proximal gastrectomy followed by esophagojejunostomy or esophagogastrostomy.
III Thoracoscopic Procedure First (Three-Step Surgery Group)
If the lymph node metastasis status in the middle or upper mediastinum needed to be examined before selecting the surgical procedures, or if transection of the esophagus was thought to be difficult using the transhiatal approach, thoracoscopy was initially performed for mediastinal lymph node dissection and transection of the esophagus. Following the abdominal procedure, either esophagojejunostomy or esophagogastrostomy was performed during a second thoracoscopic procedure, with a total of at least three steps.
IV Laparoscopy Followed by Thoracoscopy (Two-Step Surgery)
Patients in whom transection of the proximal esophagus was possible but reconstruction was not possible through the hiatus underwent initial abdominal procedures, including either total or proximal gastrectomy with systematic lymph node dissection including lower mediastinal lymphadenectomy, followed by removal of the excised specimens, if the esophagus could be divided at the cancer-free portion through the hiatus (two-step surgery group A). In the two-step groups, a gastric conduit or jejunal limb for Roux-en-Y reconstruction was prepared at the end of the first abdominal step. The remaining procedures for the second step were carried out via thoracoscopy, involving additional lower mediastinal lymph node dissection, if necessary, and reconstruction of the alimentary tract. One of the predominant reasons for three-step surgery was the limitation of the approximate setting of an endoscopic linear stapler at the proximal side of the esophagus through the hiatus during the first abdominal laparoscopic phase. To solve this problem, we developed a transthoracic endoscopic linear stapler technique in February 2013, which allowed transection of the esophagus at a cancer-negative proximal surgical margin during the first abdominal laparoscopic phase, when appropriate transection of the esophagus at the proximal side through the hiatus was impossible. Some patients who would originally have been treated by the three-step procedure therefore received the two-step procedure after February 2013. These patients who underwent transthoracic transection of the esophagus were independently classified into two-step surgery group B in this study.
V Surgical Procedures in Two-Step Surgery Groups
Two-step surgery was similar to the Ivor-Lewis procedure, but we examined the proximal surgical margins at the end of the abdominal procedure. During the transhiatal procedures, the hiatus of the diaphragm was opened vertically at the tendon center and the pleura was opened in most cases. In some cases, the diaphragm was divided on the left side to improve the operative view of the mediastinum mainly for the purpose of the reconstructive procedures, if necessary. The esophagus was divided at the cancer-free portion through the hiatus using the endoscopic linear stapler, and thoracoscopic surgery was carried out because transhiatal reconstructive procedures were considered impossible (two-step surgery A). If the esophagus could not be transected through the hiatus, the middle and lower esophagus was mobilized circumferentially, and a 12-mm trocar port was placed at the seventh or eighth intercostal space at the anterior axillary line. An endoscopic linear stapler (60-mm in length) was then inserted through the thoracic port to the esophagus, introduced into the predesigned portion and approximated, and then fired. Transhiatal reconstructive procedures were commonly impossible in these patients (two-step surgery B). At the end of the laparoscopic procedures, the gastric conduit or jejunal limb was brought up carefully until a sufficient length could be obtained for an anastomosis. In addition, prevention of internal hernia was performed by fixing transverse colon or mesocolon to the diaphragm. The patient was then placed in the prone position for the subsequent thoracoscopic procedure. Intrathoracic esophagogastrostomy and esophagojejunostomy were created by an overlapped method to make a side-to-side anastomosis, or an intrathoracic esophagogastrostomy was created by end-to-end anastomosis using a triangular stapling technique [22]. In more recent cases, the intrathoracic esophagogastrostomy was made using a double-flapped technique to prevent reflux esophagitis. During this thoracic procedure, the gastric remnant was rotated upwards to face the anastomotic aspect clearly, after fixation on the terminal side to the esophagus by sero-muscular suturing. Finally, the normal arrangement of the two organs was restored after completion of the anastomotic procedures. After thoracoscopic reconstruction of an alimentary tract in patients via two or three-step surgeries, another laparoscopic procedure was sometimes performed to make a straight alimentary tract and to ensure prevention of postoperative hiatal hernia.
VI Examination of Excised Specimens
A cancer-free proximal margin was confirmed in all patients by intraoperative examination of frozen pathological sections, as soon as the proximal surgical margin was obtained. After removal, the specimen was immediately opened longitudinally, and the lymph nodes were retrieved for pathological evaluation. The specimen was then stretched maximally and pinned to a board for gross inspection of ex vivo variables, and the specimen was then examined histologically. The length of the proximal surgical margin was described after histological examination. The tumors were staged according to the seventh edition of the TNM classification system for both gastric and esophageal cancer [23]. Some patients with clinical stage II or III according to the esophageal cancer staging system, received neoadjuvant chemotherapy to accelerate curability [24]. All patients were fully involved in the decision-making process, and informed consent was obtained from all patients. This retrospective study was approved by the Institutional Review Board of Saga University Hospital.
VII Statistical Analysis
We obtained the following clinical data from medical charts: age, sex, preoperative and pathological tumor characteristics, status of adjuvant therapy, total duration of the operation, total estimated blood loss, total number of retrieved nodes and mediastinal nodes, the incidence of complications and recurrence information. Postoperative morbidities were defined as events ≥ grade 2 according to the Clavien-Dindo classification [25].
Values are expressed as the numbers of patients or the means ± standard deviation (SD). Differences among groups were analyzed by ANOVA or χ2 tests using Fisher’s exact probability. Potential risk factors affecting the surgical step were assessed by logistic regression analysis. A two-sided P value < 0.05 was considered to be significant. Statistical analysis was performed using JMP software (SAS Institute, Cary, NC, USA).
Results
The treatment strategy and consort diagram are summarized in Figure 1. EGJC was classified as a category of Siewert type II (n = 28) or type III (n = 18) after thorough preoperative assessments with upper gastrointestinal series and endoscopic examination. All 46 patients underwent entirely laparoscopic procedures without conversion to any type of celiotomic procedure. Sixteen patients (35 %) were treated successfully by laparoscopic surgery alone (one-step surgery group), and the remaining 30 patients needed an additional thoracoscopic procedure to complete the anastomotic procedure after resection of EGJC. None of 30 patients who underwent additional thoracoscopic procedures had no conversion to conventional thoracotomy or minithoracotomy. Nine patients received three-step surgery because the esophagus at the proximal side needed to be transected at the middle portion of the thoracic esophagus, or the lymph node status needed to be determined in the mediastinum, as the lesions were spread relatively distant from the squamo-columnar junctional line or metastatic nodes were suspected preoperatively in the middle-upper mediastinum. The remaining 21 patients underwent two-step surgery.
Table 1: Patients and tumor characteristics.
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Siewert type (II / III) Gender (Female / Male) Age (years) Histology (AD / SCC / special types) Depth of tumor invasion* (T1 / T2 / T3 / T4) Lymph node metastasis* (N0 / N1 / N2 / N3) TNM stage* (I / II / III / IV) Presence of NAC |
(n = 16) 5 / 11 4 / 12 71±10
15 / 0 / 1
5 / 6 / 2 / 4
6 / 5 / 3 / 2
5 / 3 / 7 / 1 5 |
(n = 8) 3 / 5 1 / 7 71±6
7 / 1 / 0
4 / 0 / 3 / 1
4 / 1 / 2 / 1
4 / 0 / 4 / 0 1 |
(n = 13) 12 / 1 3 / 10 65±14
9 / 3 / 1
5 / 4 / 3 / 1
7 / 5 / 1 / 0
7 / 3 / 3 / 0 3 |
(n = 9) 8 / 1 3 / 6 71±9
5 / 3 / 1
6 / 1 / 1 / 1
5 / 2 / 0 / 2
5 / 1 / 2 / 1 1 |
0.0011 0.7947 0.7793
0.3126
0.3858
0.7850
0.6437 0.6468 |
Data given as number of patients or mean ± SD. TTT: technique for transthoracic transection of the esophagus during the abdominal phase. AD: adenocarcinoma. SCC: squamous cell carcinoma. NAC: neoadjuvant chemotherapy.
*Pathological staging.
#ANOVA or χ2 test.
Table 2: Surgical procedures and results.
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Type of gastrectomy and reconstruction PG JIP EG OL TST DFT others Total length of operation (min) Total blood loss (ml) Number of retrieved lymph nodes Total Mediastinal Mortality Presence of postoperative complications Respiratory Anastomotic Others Postoperative hospital stay (days) Postoperative observation period (months) Presence of recurrence |
(n = 16)
11 5 2 3 2 0 1 0 457±120 143±139
43.3±13.8 2.9±4.8 0 5 1 1 3 22±17 31±19 2 |
(n = 8)
5 3 0 3 0 0 3 0 573±147 407±603
46.1±34.2 3.5±6.0 0 2 0 1 1 58±80 41±30 1 |
(n = 13)
1 12 1 11 4 5 0 2 490±183 157±315
39.5±18.7 7.2±5.5 1 5 1 2 1 28±22 23±15 3 |
(n = 9)
2 7 0 7 1 6 0 0 537±174 99±108
37.0±17.7 9.7±8.6 0 5 3 2 0 40±29 36±31 3 |
0.0031
0.1451 0.2318
0.9452 0.0698 0.4584 0.5594 0.1045 0.7092 0.5163 0.1802 0.5681 0.5834 |
Data given as number of patients or mean ± SD. TG: total gastrectomy; PG: proximal gastrectomy; EJ: esophagojejunostomy; JIP: jejunal interposition; EG: esophagogastrostomy; OL: overlapped method; TST: triangular stapling technique; DFT: double-flapped technique.
#ANOVA or χ2 test.
Table 3: Results of examination of excised specimens
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Maximal tumor size (cm) Length of esophageal invasion (cm) Proximal margin (cm) Distal margin (cm) Length of excised mediastinal organs (cm) |
(n = 16) 4.3±2.9 0.9±0.5 2.1.±1.6 11.5±5.9 2.3±1.4 |
(n = 8) 4.4±1.9 2.6±1.4 2.9±1.5 14.3±7.9 4.3±2.1 |
(n = 13) 4.6±2.9 4.0±1.6 2.9±1.8 9.8±4.2 8.1±3.2 |
(n = 9) 3.2±1.9 3.2±0.9 3.5±1.4 10.6±6.6 6.7±1.9 |
0.6583 <0.0001 0.2279 0.6168 <0.0001 |
Data given as mean ± SD.
#ANOVA.
I Patient Characteristics and Surgical Results
The patient and tumor characteristics according to the type of surgery are summarized in Tables 1 and 2. The type of gastrectomy and number of surgical steps were significantly associated with Siewert classification. Of the 28 patients with a Siewert type II tumors, three underwent total gastrectomy with transhiatal esophagojejunostomy by one-step surgery (n = 2) or intrathoracic esophagojejunostomy by three-step surgery (n = 1), and 23 patients were treated with proximal gastrectomy with esophagogastrostomy by one-step surgery (n = 2) two-step surgery A (n = 3), two-step surgery B (n = 11), or three-step surgery (n = 7). The remaining three patients with Siewert type II tumors underwent jejunal interposition after proximal gastrectomy by one- or two-step surgery (n = 1 and 2, respectively). Of the 18 patients with Siewert type III tumors, two underwent proximal gastrectomy under a laparoscope alone, and the remaining 16 patients underwent laparoscopic esophagojejunostomy after total gastrectomy by one-step surgery (n = 9), thoracoscopic esophagojejunostomy after total gastrectomy by two-step surgery A (n = 5) or two-step surgery B (n = 1), or by three-step surgery (n = 1). Transhiatal reconstruction of the alimentary tract could not be carried out in any patients undergoing two- or three-step surgery because of the short remnant esophagus. Overall, patients with Siewert type III tumors treated by total gastrectomy and esophagojejunostomy were more likely to undergo one step-surgery. The incidences of Siewert type II or III (P = 0.0011), total or proximal gastrectomy (P = 0.0031), and esophagojejunostomy or esophagogastrostomy (P = 0.0013) differed significantly among the four surgical groups, but there were no significant differences in terms of gender, age, histology, TNM staging, or use of neoadjuvant chemotherapy.
The surgical procedures and outcomes of the 46 patients are summarized in Table 2. The total duration of the operation included the duration of the positional changes in this study. Although one-step surgery was the shortest procedure in the current study, the difference was not significant. There was no significant difference in estimated blood loss among the four surgical groups. The type of gastrectomy and extent of mediastinal lymph node dissection were associated with the total number of retrieved lymph nodes. The numbers of total and mediastinal lymph nodes, therefore differed between three-step and one-step surgery groups. However, there was no significant difference in retrieved lymph nodes among the four surgical groups.
Figure 2: Examination of excised specimens: (A) Length of esophageal invasion by the tumor; (B) Length of the proximal surgical margin; (C) Length of the excised mediastinal organs.
II Examination of Excised Specimens
The results of examinations of the maximally stretched excised specimens in each surgical group are shown in Table 3. No additional esophageal resection was performed in the thoracic phase after the prior laparoscopic procedures, because transection successfully achieved a cancer-negative proximal surgical margin. There were no significant differences in tumor size among the groups. The length of tumor invasion of the esophagus was significantly shorter in the one-step surgery group who underwent the laparoscopic procedure alone, compared with the other surgical groups. Intraoperative pathological examination of frozen sections was carried out to confirm a cancer-negative proximal surgical margin in all 46 patients. However, one patient in the two–step surgery group B had a cancer-positive proximal margin at the final hematoxylin and eosin pathological diagnosis, despite a negative margin during intraoperative pathological examination. The ex vivo proximal surgical margin was longest in the three-step group, but the difference was not significant. We measured the lengths of the ex vivo excised mediastinal organs, which ranged from the lower portion of the actual esophageal hiatus to the proximal surgical margin on the excised specimens and were composed of a possibly sliding proximal portion of the stomach and a distal portion of the esophagus (Figure 2). The length of the excised mediastinal organs in the two-step surgery B group was similar to that in the three-step group and was much longer than in the one- or two-step surgery A groups (P < 0.0001). The extent to which the stomach slid into the mediastinum, i.e., the length of the sliding hiatal hernia, could be calculated by the length of the excised mediastinal organs minus the proximal margin, minus the length of the esophageal invasion by the tumor. There was no significant difference in the lengths among the four groups.
Table 4: Results of univariate and multivariate logistic regression analyses of risk factors for multi-step surgery.
|
Variables |
One-step |
Multi-step* |
Univariate analyses |
Multivariate analyses |
||||
|
|
(n = 16) |
(n = 30) |
Odds |
95% CI |
P |
Odds |
95% CI |
P |
|
Age (years) |
71±10 |
69±11 |
0.978 |
0.921-1.038 |
0.4537 |
|
|
|
|
Gender (M/F) |
12 / 4 |
23 / 7 |
1.095 |
0.267-4.500 |
0.8996 |
|
|
|
|
Histology (SCC/AD +others) |
0 / 16 |
8 / 22 |
4.565 |
0.509-40.961 |
0.1749 |
|
|
|
|
Siewert type (III/II) |
11 / 5 |
7 / 23 |
0.138 |
0.036-0.536 |
0.0042 |
0.069 |
0.003-1.861 |
0.1119 |
|
pT (pT2-4/pT1) |
12 / 5 |
15 / 15 |
0.381 |
0.100-1.455 |
0.1580 |
|
|
|
|
pN (pN1-3/pN0) |
10 / 6 |
14 / 16 |
0.488 |
0.140-1.700 |
0.2596 |
|
|
|
|
pStage (III-IV/I-II) |
8 / 8 |
10 / 20 |
0.389 |
0.112-1.352 |
0.1373 |
|
|
|
|
NAC (+/-) |
5 / 11 |
5 / 25 |
0.440 |
0.105-1.835 |
0.2599 |
|
|
|
|
Gastrectomy type (TG/PG) |
11 / 5 |
8 / 22 |
0.165 |
0.044-0.626 |
0.0080 |
|
|
|
|
Reconstruction type (EJ/EG) |
13 / 3 |
9 / 21 |
0.099 |
0.023-0.434 |
0.0022 |
0.049 |
0.002-1.564 |
0.0877 |
|
Operative time (min) |
457±120 |
526±170 |
1.003 |
0.999-1.008 |
0.1608 |
|
|
|
|
Blood loss (ml) |
143±139 |
206±385 |
1.001 |
0.998-1.003 |
0.5371 |
|
|
|
|
Total LN |
43.3±13.8 |
41.8±22.3 |
0.993 |
0.964-1.023 |
0.6530 |
|
|
|
|
Mediastinal LN |
2.9±4.8 |
6.9±6.9 |
1.146 |
0.998-1.317 |
0.0541 |
|
|
|
|
Maximal tumor size (cm) |
4.3±2.9 |
4.1.±2.4 |
0.998 |
0.974-1.022 |
0.8543 |
|
|
|
|
Length of esophageal invasion (cm) |
0.9±0.5 |
3.5.±1.5 |
1.193 |
1.068-1.334 |
0.0018 |
1.271 |
1.042-1.549 |
0.0177 |
|
Proximal margin (cm) |
2.1.±1.6 |
3.1.±1.6 |
1.047 |
0.998-1.098 |
0.0593 |
|
|
|
|
Distal margin (cm) |
11.5±5.9 |
11.2.±6.0 |
0.999 |
0.988-1.010 |
0.8643 |
|
|
|
|
Length of excised mediastinal organs (cm) |
2.3±1.4 |
6.7.±2.9 |
1.121 |
1.044-1.203 |
0.0017 |
|
|
|
*Multi-step surgery group included two-step surgery groups A and B, and three-step surgery group.
III Postoperative Outcomes
In-hospital mortality occurred in one patient in the two-step surgery group B who had a cancer-positive surgical margin and who died of pulmonary edema, probably caused by the intense preoperative induction chemotherapy performed with the aim of down-staging the tumor. Postoperative morbidities were more common after two- and three-step surgeries compared with after one-step surgery, but the difference was not significant (Table 2). Respiratory complications were not often observed in any surgery group. Anastomotic leakage occurred in one patient with jejunal interposition reconstruction after proximal gastrectomy in the one-step surgery group, in one patient with intrathoracic esophagojejunostomy in the two-step surgery group A and in two patients each with intrathoracic esophagogastrostomy in the two-step surgery group B and three-step surgery group. The rate of anastomotic failure after intrathoracic esophagogastrostomy was 19% compared with 8% for esophagojejunostomy in the current series (P = 0.3893). The types of esophagogastrostomy that were resulted in postoperative anastomotic leakage were the overlapped method (n = 2), the triangular stapling technique (n = 1) and another hand-sewing end-to end anastomosis (n = 1). Esophagogastrostomy with a double flapped technique was performed recently in one case transhiatally and in three cases transthoracically, with no anastomotic leakage. The length of postoperative hospital stay was prolonged as a result of postoperative complications, especially anastomotic failure: however, the postoperative hospital stay was shortest in the one-step surgery group, but there was no significant difference among the groups. During the postoperative observation periods, recurrent disease was observed in nine patients with T2-T4 tumors and in one patient with a pathological T1b N1-tumor. The recurrence sites were hematogenous metastasis in the liver or lung (n = 5), peritoneal dissemination (n = 3) and recurrence in the paraaortic lymph nodes (n = 1). Locoregional recurrence, including at the anastomosis, has not been observed so far. There were no significant differences in recurrence rates among the four surgical groups.
IV Risk Factors for Requiring Multi-Step Surgery
Unadjusted regression analyses of all 46 patients showed that Siewert type, proximal or total gastrectomy, esophagogastrostomy or esophagojejunostomy, length of esophageal invasion, and length of excised mediastinal organs were significant risk factors for requiring multi-step surgery. Few patients underwent jejunal interposition reconstruction after proximal gastrectomy, and type of reconstruction was closely associated with the type of gastrectomy. The length of excised mediastinal organs depended on the extent of esophageal invasion. After exclusion of these confounding factors, multivariate regression analysis showed that only the length of esophageal invasion was an independent risk factor for requiring multi-step surgery (95% confidence interval, 1.042-1.549; odds ratio, 1.271; P = 0.0177) (Table 4).
Discussion
In this study, we demonstrated that obtaining an optimal proximal surgical margin by MIS in patients with Siewert type II or III EGJC was complicated, while the extents of gastrectomy and lymph node dissection were associated with the location and staging of the tumor. Only 35 % of patients with EGJC were successfully treated by laparoscopic surgery alone in our institutes, and the most significant risk factor for requiring multi-step surgery was longer esophageal invasion of the tumor which was associated with longer excised mediastinal organs.
This study had some potential limitations or bias in relation to surgical strategies, patient selections, and technical learning curves. However, the surgical strategies for EGJC were consistent throughout during the study period in our institutes, and all patients with EGJC were treated by MIS, except for three cases who underwent previous major celiotomic surgery and two who were treated with planned subtotal esophagectomy and cervical anastomosis. Uniformity among the proximal surgical margins among the four surgical groups suggested little bias in terms of the surgical strategy. Regarding technical skills and learning curves, we have performed laparoscopic gastrectomy for gastric cancer in our institutes since 1996 and thoracoscopic esophagectomy since 1998 and thus had extensive experience of MIS for both esophageal and gastric cancer before starting MIS for EGJC [2-4]. In addition, if there had been strong bias among surgeon was strong, logistic regression would not have identified any factor being associated with requiring multi-step surgery.
We consider that a cancer-free proximal margin is vital in the surgical treatment of Siewert types II and III EJGC. The proximal resection margin is known to have a major impact on the survival of patients with EGJC. In a series of 505 patients who underwent EGJC surgery, Barbour et al. showed a significant survival benefit of grossly negative ex vivo esophageal proximal surgical margins > 3.8 cm and R0 resection with ≥ 15 removed lymph nodes, though the benefit was limited to patients with ≥ T2 tumors and six or fewer positive lymph nodes [23]. In a review of 140 cases, Mine et al. revealed that gross proximal margin lengths of > 2.0 cm in resected specimens seemed to be satisfactory for patients with type II and III adenocarcinoma of the esophagogastric junction treated by transhiatal gastrectomy [19]. Some researchers demonstrated that positive proximal margins could be avoided by resecting ≥ 10 cm of macroscopically tumor-free esophagus because of the possibility of direct submucosal tumor invasion or lymphatic permeation [26-28]. However, the optimal proximal surgical margins in EGJC remains uncertain because the ways in which the ex vivo measurements of the excised specimens were made differed among studies and considering the serious shrinkage of the excised esophagus. We tried to obtain macroscopic surgical margins > 2.0 cm and cancer-negative margin by frozen pathological examination in this series. No locoregional recurrence had been observed at the median postoperative observation period of 51 months.
In this series, a sufficient length of proximal margin could be obtained in patients with Siewert type II or III tumors by transection of the proximal esophagus through the hiatus using the laparoscopic approach alone, if the length of esophageal invasion by the tumor was small. However, transhiatal transection of the esophagus was difficult in patients with mild esophageal tumor invasion and a serious sliding hiatal hernia or a large tumor. The present transthoracic transection technique enabled us to achieve similar proximal margins to those obtained during three-step surgery. On one patient treated with transthoracic transection who had undergone intense induction chemotherapy prior to surgery had a positive proximal margin according to the postoperative permanent histological diagnosis, despite a cancer-negative intraoperative pathological diagnosis by frozen section studies. However, we considered that this case was exceptional. We measured the lengths of the excised mediastinal organs which were composed of a sliding proximal portion of the stomach and a distal portion of the esophagus. The mean ex vivo length using the present transthoracic transection technique was 8.1 cm, which was similar to that achieved by three-step surgery. Considering shrinkage of the excised esophagus an ex vivo length of 8.1 cm may correspond to > 10 cm in situ. During the abdominal phase when a patient is placed in the supine position, the maximal level of transection of the esophagus is limited by the anatomy of the inferior pulmonary veins [29]. In contrast, transection of the esophagus during a thoracoscopic right thoracic approach is not affected by any anatomical structures, irrespective of the position (prone or lateral decubitus) during the thoracoscopic phase. A transthoracic approach prior to the abdominal phase may thus be optimal for surgical treatment in some cases of EGJC. Even if a gross proximal margin > 2.0 cm is safe, transection of the esophagus at the appropriate proximal side through the hiatus during the abdominal phase as the first step of MIS cannot be performed easily in a patient with a sliding hiatal hernia.
However, three steps were always required to complete these procedures, unless the excised specimen was removed through an additionally-created thoracotomy or minithoracotomy during the thoracic phase as the second step after the abdominal laparoscopic phase. The transthoracic transection technique is safe and feasible and helps to ensure successful transection of the esophagus at a proximal cancer-negative margin during the first abdominal phase in MIS. This resulted in a reduction in patient-positioning changes, which was one of the most time-consuming procedures requiring 20-30 minutes to move a patient from a supine to a prone or lateral decubitus position. Moreover, avoiding the thoracoscopic procedure might help to reduce respiratory complications and shorten the operation. In this series, three of nine patients who underwent three-step surgery developed respiratory disorders (33%), compared with only one patient each in the one- and two-step surgery groups (6% and 5%, respectively). However, this difference was not significant, probably because of the small number of patients in each group. In open surgery, transthoracic procedures are associated with increased morbidity and mortality compared with transabdominal and transhiatal approaches [30-32]. We expected that the decreased invasiveness provided by MIS may be associated with fewer respiratory complications. However, repeated thoracoscopic procedures might be harmful and should be avoided. Previous reports found that 7%–37 % of patients with advanced gastric cancer invading the lower esophagus had metastatic lymph nodes in the lower mediastinum [9, 13-15]. Although a previous randomized controlled study of open surgery demonstrated that an additional thoracotomic approach had no survival benefit in patients with Siewert type II or III tumors with esophageal invasion was ≤ 3 cm [15], other researchers have suggested that curative resection of tumors invading the esophagus by > 3 cm should be accompanied by lower mediastinal lymph node dissection [15, 33]. Considering the variety of surgical strategies used to treat EGJC, the extent of lymph node dissection should be decided on an individual bias, according to the tumor location and staging. In the current series, lymphadenectomy could be performed sufficiently below the inferior pulmonary veins by the transhiatal approach.
Failure of the intrathoracic anastomosis, mainly by intrathoracic esophagogastrostomy, is relatively common despite a cancer-free proximal surgical margin. In the current study, creation of an esophagogastrostomy between a short esophageal remnant and relatively large gastric remnant was more difficult transhiatally compared with esophagojejunostomy, and transthoracic anastomosis or jejunal interposition reconstruction was consequently required. Jejunal interposition may be useful but is complicated in MIS because it requires the creation of at least three anastomoses [34]. Moreover, the reservoir function of the gastric remnant is uncertain or may be spoiled in this type of reconstruction. Regarding intrathoracic anastomosis, we previously reported the success of intrathoracic esophagojejunostomy during the initial seven cases treated with MIS however, the rate of anastomotic failure after intrathoracic esophagogastrostomy was 19% compared with 8% for esophagojejunostomy in the current series [7]. The intrathoracic overlapped esophagogastrostomy during the initial period of this study, might have interrupted the blood supply of the gastric conduit around the anastomosis. We therefore subsequently created end-to-end esophagogastrostomy using a triangular stapling technique, which is commonly used for anastomosis at the neck after subtotal esophagectomy however, this did not reduce the incidence of anastomotic failure because of the complicated procedure in the thorax [22]. In addition, end-to-end esophagogastrostomy using a triangular stapling technique does not prevent regurgitation, which may occur in the event of anastomosis at the middle –lower mediastinum. We recently developed esophagogastrostomy using a double-flapped technique. The reconstruction method in the abdominal procedure has been well documented in patients treated by laparoscopic proximal gastrectomy [35]. However, in contrast to esophagogastrostomy in the abdomen, esophagogastrostomy using a double-flapped technique in the thorax, is more complicated because the reverse arrangement between the gastric remnant and the esophagus due to the prone positioning, and because the operative view does not face directly from the right transthoracic side. The anastomosis must therefore be created upside down during the common abdominal procedure in the supine position and then reversed. Intrathoracic or transhiatal esophagogastrostomy with a double-flapped technique seems to be complicated but has advantageous in terms of reducing anastomotic leakage and preventing reflux esophagitis. A previous case report demonstrated an advantage of transhiatal esophagogastrostomy with a double-flapped technique for EGJC, and the results in the current series also suggested that this technique had actual benefits [36].
Conclusion
We herein showed that multi-step procedures may be needed to achieve a cancer-negative proximal margin followed by safe and successful alimentary reconstruction in some patients with Siewert type II or III EGJC undergoing MIS. We also demonstrated that the need for multi-step procedures was closely associated with the length of esophageal invasion of the tumor. It is necessary to develop easier and more reliable procedures minimize invasiveness during surgery for EGJC.
Acknowledgements
We thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflicts of interest
None.
Article Info
Article Type
Original ResearchPublication history
Received: Mon 21, Oct 2019Accepted: Fri 15, Nov 2019
Published: Thu 12, Dec 2019
Copyright
© 2023 Hirokazu Noshiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.04.02
Author Info
Hirokazu Noshiro Yukie Yoda Hironori Iwasaki Taketo Matsunaga Akihiko Uchiyama
Corresponding Author
Hirokazu NoshiroDepartment of Surgery, Faculty of Medicine, Saga University, Saga, Japan
Figures & Tables
Table 1: Patients and tumor characteristics.
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Siewert type (II / III) Gender (Female / Male) Age (years) Histology (AD / SCC / special types) Depth of tumor invasion* (T1 / T2 / T3 / T4) Lymph node metastasis* (N0 / N1 / N2 / N3) TNM stage* (I / II / III / IV) Presence of NAC |
(n = 16) 5 / 11 4 / 12 71±10
15 / 0 / 1
5 / 6 / 2 / 4
6 / 5 / 3 / 2
5 / 3 / 7 / 1 5 |
(n = 8) 3 / 5 1 / 7 71±6
7 / 1 / 0
4 / 0 / 3 / 1
4 / 1 / 2 / 1
4 / 0 / 4 / 0 1 |
(n = 13) 12 / 1 3 / 10 65±14
9 / 3 / 1
5 / 4 / 3 / 1
7 / 5 / 1 / 0
7 / 3 / 3 / 0 3 |
(n = 9) 8 / 1 3 / 6 71±9
5 / 3 / 1
6 / 1 / 1 / 1
5 / 2 / 0 / 2
5 / 1 / 2 / 1 1 |
0.0011 0.7947 0.7793
0.3126
0.3858
0.7850
0.6437 0.6468 |
Data given as number of patients or mean ± SD. TTT: technique for transthoracic transection of the esophagus during the abdominal phase. AD: adenocarcinoma. SCC: squamous cell carcinoma. NAC: neoadjuvant chemotherapy.
*Pathological staging.
#ANOVA or χ2 test.
Table 2: Surgical procedures and results.
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Type of gastrectomy and reconstruction PG JIP EG OL TST DFT others Total length of operation (min) Total blood loss (ml) Number of retrieved lymph nodes Total Mediastinal Mortality Presence of postoperative complications Respiratory Anastomotic Others Postoperative hospital stay (days) Postoperative observation period (months) Presence of recurrence |
(n = 16)
11 5 2 3 2 0 1 0 457±120 143±139
43.3±13.8 2.9±4.8 0 5 1 1 3 22±17 31±19 2 |
(n = 8)
5 3 0 3 0 0 3 0 573±147 407±603
46.1±34.2 3.5±6.0 0 2 0 1 1 58±80 41±30 1 |
(n = 13)
1 12 1 11 4 5 0 2 490±183 157±315
39.5±18.7 7.2±5.5 1 5 1 2 1 28±22 23±15 3 |
(n = 9)
2 7 0 7 1 6 0 0 537±174 99±108
37.0±17.7 9.7±8.6 0 5 3 2 0 40±29 36±31 3 |
0.0031
0.1451 0.2318
0.9452 0.0698 0.4584 0.5594 0.1045 0.7092 0.5163 0.1802 0.5681 0.5834 |
Data given as number of patients or mean ± SD. TG: total gastrectomy; PG: proximal gastrectomy; EJ: esophagojejunostomy; JIP: jejunal interposition; EG: esophagogastrostomy; OL: overlapped method; TST: triangular stapling technique; DFT: double-flapped technique.
#ANOVA or χ2 test.
Table 3: Results of examination of excised specimens
|
Surgical group |
One-step |
Two-step A (without TTT) |
Two-step B (with TTT) |
Three-step |
P# |
|
Maximal tumor size (cm) Length of esophageal invasion (cm) Proximal margin (cm) Distal margin (cm) Length of excised mediastinal organs (cm) |
(n = 16) 4.3±2.9 0.9±0.5 2.1.±1.6 11.5±5.9 2.3±1.4 |
(n = 8) 4.4±1.9 2.6±1.4 2.9±1.5 14.3±7.9 4.3±2.1 |
(n = 13) 4.6±2.9 4.0±1.6 2.9±1.8 9.8±4.2 8.1±3.2 |
(n = 9) 3.2±1.9 3.2±0.9 3.5±1.4 10.6±6.6 6.7±1.9 |
0.6583 <0.0001 0.2279 0.6168 <0.0001 |
Data given as mean ± SD.
#ANOVA.
Table 4: Results of univariate and multivariate logistic regression analyses of risk factors for multi-step surgery.
|
Variables |
One-step |
Multi-step* |
Univariate analyses |
Multivariate analyses |
||||
|
|
(n = 16) |
(n = 30) |
Odds |
95% CI |
P |
Odds |
95% CI |
P |
|
Age (years) |
71±10 |
69±11 |
0.978 |
0.921-1.038 |
0.4537 |
|
|
|
|
Gender (M/F) |
12 / 4 |
23 / 7 |
1.095 |
0.267-4.500 |
0.8996 |
|
|
|
|
Histology (SCC/AD +others) |
0 / 16 |
8 / 22 |
4.565 |
0.509-40.961 |
0.1749 |
|
|
|
|
Siewert type (III/II) |
11 / 5 |
7 / 23 |
0.138 |
0.036-0.536 |
0.0042 |
0.069 |
0.003-1.861 |
0.1119 |
|
pT (pT2-4/pT1) |
12 / 5 |
15 / 15 |
0.381 |
0.100-1.455 |
0.1580 |
|
|
|
|
pN (pN1-3/pN0) |
10 / 6 |
14 / 16 |
0.488 |
0.140-1.700 |
0.2596 |
|
|
|
|
pStage (III-IV/I-II) |
8 / 8 |
10 / 20 |
0.389 |
0.112-1.352 |
0.1373 |
|
|
|
|
NAC (+/-) |
5 / 11 |
5 / 25 |
0.440 |
0.105-1.835 |
0.2599 |
|
|
|
|
Gastrectomy type (TG/PG) |
11 / 5 |
8 / 22 |
0.165 |
0.044-0.626 |
0.0080 |
|
|
|
|
Reconstruction type (EJ/EG) |
13 / 3 |
9 / 21 |
0.099 |
0.023-0.434 |
0.0022 |
0.049 |
0.002-1.564 |
0.0877 |
|
Operative time (min) |
457±120 |
526±170 |
1.003 |
0.999-1.008 |
0.1608 |
|
|
|
|
Blood loss (ml) |
143±139 |
206±385 |
1.001 |
0.998-1.003 |
0.5371 |
|
|
|
|
Total LN |
43.3±13.8 |
41.8±22.3 |
0.993 |
0.964-1.023 |
0.6530 |
|
|
|
|
Mediastinal LN |
2.9±4.8 |
6.9±6.9 |
1.146 |
0.998-1.317 |
0.0541 |
|
|
|
|
Maximal tumor size (cm) |
4.3±2.9 |
4.1.±2.4 |
0.998 |
0.974-1.022 |
0.8543 |
|
|
|
|
Length of esophageal invasion (cm) |
0.9±0.5 |
3.5.±1.5 |
1.193 |
1.068-1.334 |
0.0018 |
1.271 |
1.042-1.549 |
0.0177 |
|
Proximal margin (cm) |
2.1.±1.6 |
3.1.±1.6 |
1.047 |
0.998-1.098 |
0.0593 |
|
|
|
|
Distal margin (cm) |
11.5±5.9 |
11.2.±6.0 |
0.999 |
0.988-1.010 |
0.8643 |
|
|
|
|
Length of excised mediastinal organs (cm) |
2.3±1.4 |
6.7.±2.9 |
1.121 |
1.044-1.203 |
0.0017 |
|
|
|
*Multi-step surgery group included two-step surgery groups A and B, and three-step surgery group.

References
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS et al. (2003) Minimally invasive esophagectomy. Outcomes in 222 patients. Ann Surg 238: 486-494. [Crossref]
- Noshiro H, Nagai E, Shimizu S, Uchiyama A, Kojima M et al. (2007) Minimally invasive radical esophagectomy for esophageal cancer. Esophagus 4: 59-65.
- Shimizu S, Noshiro H, Nagai E, Uchiyama A, Tanaka M et al. (2003) Laparoscopic gastric surgery in a Japanese institution: analysis of the initial 100 procedures. J Am Coll Surg 197: 372-378. [Crossref]
- Noshiro H, Nagai E, Shimizu S, Uchiyama A, Tanaka M et al. (2005) Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 19: 1592-1596. [Crossref]
- Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K et al. (2009) Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg 144: 1138-1142. [Crossref]
- Hong L, Zhang Y, Zhang H, Yang J, Zhao Q (2013) The short-term outcome of three-field minimally invasive esophagectomy for Siewert type I esophagogastric junctional adenocarcinoma. Ann Thorac Surg 96: 1826-1831. [Crossref]
- Noshiro H, Miyasaka Y, Akashi M, Iwasaki H, Ikeda O et al. (2012) Minimally invasive esophagogastrectomy for esophagogastric junctional cancer. Ann thorac Surg 93: 214-220. [Crossref]
- Crew KD, Neugut AI (2004) Epidemiology of upper gastrointestinal malignancies. Semin Oncol 31: 450-464. [Crossref]
- Steevens J, Botterweck AA, Dirx MJ, van der Brandt PA, Schouten LJ (2010) Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol 22: 669-678. [Crossref]
- Kauppila JH, Lagergren J (2016) The surgical management of esophago-gastric junctional cancer. Surg Oncol 25: 394-400. [Crossref]
- Wei MT, Zhang YC, Deng XB, Yang TH, He YZ et al. (2014) Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: a meta-analysis. World J Gastroenterol 20: 10183-10192. [Crossref]
- Haverkamp L, Ruurda JP, van Leeuwen MS, Siersema PD, van Hillegersberg R (2014) Systematic review of the surgical strategies of adenocarcinomas of the gastroesophageal junction. Surg Oncol 23: 222-228. [Crossref]
- Clark GW, Peters JH, Ireland AP, Ehsan A, Hagen JA et al. (1994) Nodal metastasis and sites of recurrence after en bloc esophagectomy for adenocarcinoma. Ann Thorac Surg 58: 646-653. [Crossref]
- Husemann B (1989) Cardia carcinoma considered as a distinct clinical entity. Br J Surg 76: 136-139. [Crossref]
- Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K et al. (2006) Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 7: 644-651. [Crossref]
- Ito H, Clancy TE, Osteen RT, Swanson RS, Bueno R et al. (2004) Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg 199: 880-886. [Crossref]
- Wayman J, Dresner SM, Raimes SA, Griffin SM (1999) Transhiatal approach to total gastrectomy for adenocarcinoma of the gastric cardia. Br J Surg 86: 536-540. [Crossref]
- Mine S, Sano T, Hiki N, Yamada K, Kosuga T et al. (2013) Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg 100: 1050-1054. [Crossref]
- Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS et al. (2007) Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 246: 1-8. [Crossref]
- Siewert JR, Stein HJ (1996) Carcinoma of the cardia: carcinoma of the gastroesophageal junction-classification, pathology and extent of resection. Dis Esophagus 9: 173-182. [Crossref]
- Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N et al. (2017) Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 20: 69-83. [Crossref]
- Noshiro H, Urata M, Ikeda O, Iwasaki H, Nabae T et al. (2013) Triangulating stapling technique for esophagogastrostomy after minimally invasive esophagectomy. Surgery 154: 604-610. [Crossref]
- Sobin LH, Gospodarowicz MK, Wittekind CH (2009) TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell.
- O'Neill JR, Kennedy ED, Save V, Langdale-Brown B, Wall L et al. (2017) Patients unfit for neoadjuvant therapy may still undergo resection of locally advanced esophageal or esophagogastric junctional cancer with acceptable oncological results. Int J Surg Oncol (NY) 2: e09. [Crossref]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D et al. (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250: 187-196. [Crossref]
- Papachristou DN, Agnanti N, D’Agostino H, Fortner JG (1980) Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg 139: 711-713. [Crossref]
- Bozzetti F, Bignami P, Bertario L, Fissi S, Eboli M (2000) Surgical treatment of gastric cancer invading the oesophagus. Eur J Surg Oncol 26: 810-814. [Crossref]
- Sakaguchi T, Watanabe A, Sawada H, Yamada Y, Tatsumi M et al. (1998) Characteristics and clinical outcome of proximal-third gastric cancer. J Am Coll Surg 187: 352-357. [Crossref]
- Siu KF, Cheung HC, Wong J (1986) Shrinkage of the esophagus after resection for carcinoma. Ann Surg 203: 173-176. [Crossref]
- Meyer W, Popp M, Klinger L, Awad-Allah A, Gebhardt C (2002) Results of surgical therapy of adenocarcinomas of the esophagogastric junction according to a standardized surgical resection technique. Dig Surg 19: 269-274. [Crossref]
- Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD et al. (2004) The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 198: 42-50. [Crossref]
- Siewert JR, Feith M, Werner M, Stein HJ (2000) Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 232: 353-361. [Crossref]
- Nunobe S, Ohyama S, Sonoo H, Hiki N, Fukunaga T et al. (2008) Benefit of mediastinal and para-aortic lymph-node dissection for advanced gastric cancer with esophageal invasion. J Surg Oncol 97: 392-395. [Crossref]
- Stein HJ, Feith M, Mueller J, Werner M, Siewert JR (2000) Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg 232: 733-742. [Crossref]
- Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S Kagawa S et al. (2016) Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg 223: e7-e13. [Crossref]
- Hosoda K, Yamashita K, Moriya H, Washio M, Mieno H et al. (2018) Esophagogastric junction cancer successfully treated by laparoscopic proximal gastrectomy and lower esophagectomy with intrathoracic double-flap technique: A case report. Asian J Endosc Surg 11: 160-164. [Crossref]
