Renal Pelvis and Renal Calices Sarcomatoid Carcinoma: A Case Report
A B S T R A C T
Introduction: Sarcomatoid carcinoma is one of the malignant neoplasms characterized by the ability to differentiate into both epithelial tissue and mesenchymal tissue. And it is rare in urinary system with a high degree of malignancy and prognosis is poor.
Patient Concerns: The present article reports a 63-year-old man presented with recurrent left flank pain for two years. Radiological tests suggested a space-occupying lesion in left kidney with enlarged paranephric /retroperitoneal lymph nodes.
Diagnosis: The pathological diagnosis following left nephrectomy indicated renal pelvis and renal calices sarcomatoid carcinoma; pTNM: T4N0M0.
Interventions: No radiotherapy or chemotherapy were performed after the surgery and underwent rapid growth.
Outcomes: The patients died six months later.
Conclusion: Renal pelvis and renal calices sarcomatoid carcinomas is a highly malignant tumor and difficult to diagnose at its early stage. Definite diagnosis relies on the combination of immunohistochemistry (IHC) and morphological analyses and there currently is no effective treatment.
Keywords
Sarcomatoid carcinoma, Renal pelvis, renal calices, immunohistochemistry, treatment
Introduction
Renal pelvis and renal calices sarcomatoid carcinoma is very rare in the clinical practice. The incidence of this disease is unclear. According to our literature search, less than 30 cases of sarcomatoid carcinoma was reported until now [1-3]. Thus, it is impossible to summarize the character of this disease systematically. One renal pelvis and renal calices sarcomatoid carcinoma patient was presented in our center in 2015. The objective of this article was to describe the clinical features of this case and review the pathology, immunohistochemistry (IHC), treatment and prognosis in accordance with the available literature.
Case Report
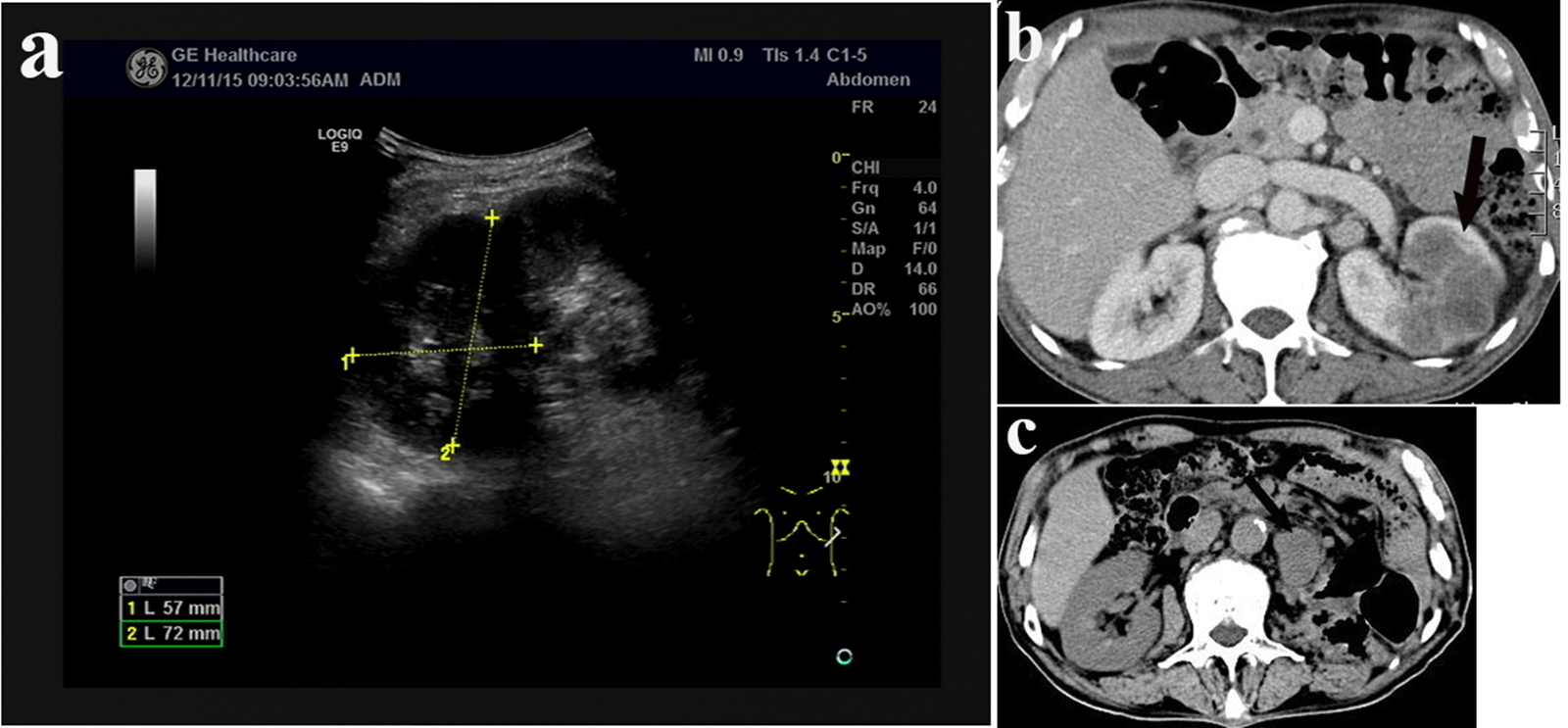
A 63-year-old man presented with recurrent left flank pain for two years and aggravated for one month was referred to our medical center on 9th Dec 2015.There were no symptoms with fever, frequent micturition, the urgency of urination, the urine pain and gross hematuria. PE: there was tenderness in left lumbar region. No abdominal mass was accessed. Radiological tests suggested space-occupying lesion in left kidney with enlarged paranephric/retroperitoneal lymph nodes (Figures 1a & 1b). Renal needle biopsy suggested high grade renal carcinoma. IHC panel: Vimentin (+), CK-pan (+), CK7 (+), CD 10 (+), PAX-2 (±), SMA (-), CD34 (-), HMB45 (-) and P63 (-). Left nephrectomy was performed on 18th Dec 2015. The tumor size was about 6*4*4 cm and gray in colour. Mucous membrane of renal pelvis was smooth. The pathological diagnosis following left nephrectomy indicated renal pelvis and renal calices sarcomatoid carcinoma; pTNM: T4N0M0. IHC panel: CK-pan (+), CK7 (partially +), Vimentin (partially +), CD10 (sporadic +) and P63 (-). After the consultation with the pathologist from Peking Union Medical College Hospital, renal cell carcinoma was excluded, and we decided that the diagnosis was undifferentiated sarcomatoid carcinoma. No radiotherapy or chemotherapy was performed after the surgery. Radiological tests on 18th Mar 2016 suggested the recurrence of tumor (Figure 1c). According to the desire of the patient’s family, no additional treatment was performed. And the patients died six months later.
Figure 1: a) Intravenous urogram indicate that left kidney is non-significantly enhanced. Kidney size is 27*30 mm. The enhancement of the lesion decreased slightly in the venous phase. Space-occupying lesion could be identified, and renal cancer could not be excluded. b) Renal CT plain scan and three phases enhancement scan show untypical hypodense shadow in the middle-upper end of the left kidney. The largest section is about 5.6*4.6 cm. CT-value is about 30HU. The density of lesion is low in either of the three phrases. The boundary is unclear. These all indicate space-occupying lesion while renal cancer could not be excluded. Enlarged paranephric/retroperitoneal lymph nodes could also be found. c) CT scan shows that after left nephrectomy and lienectomy, the local connective tissue was unclear, and multiple nodules could be found. Tumor recurrence or metastasis could not be excluded.
Discussion
The incidence of renal pelvis and renal calices sarcomatoid carcinoma is low. The reported cases indicated that the ratio of male patient/female patient is 2:1~3:1, and the older patients, right side lesion are more commonly seen [1-3]. There is no specific clinical manifestation indicating the onset of renal pelvis and renal calices sarcomatoid carcinoma. Some non-specific manifestations include gross hematuria and flank pain/ swelling and uronephrosis [1-6]. The early diagnosis is quite difficult for this disease. The sensitivity of radiological tests limits the chance of discovering the tumor in early stage. Radiological manifestation usually indicates the inferior TNM staging. Definite diagnosis relies on pathological test and immunohistochemistry following nephrectomy and percutaneous renal biopsy. There is no treatment guideline available for this disease. Nephrectomy is performed in most of the patients, while chemotherapy and radiotherapy after nephrectomy are less commonly used [2, 4, 6, 7]. The available evidence suggests that chemotherapy and radiotherapy have little effect on long term survival rate [2, 6]. Prognosis of this disease is poor. Most patients die in the first two years after the diagnosis. TNM stage of the neoplasm is associated with the prognosis [7, 8].
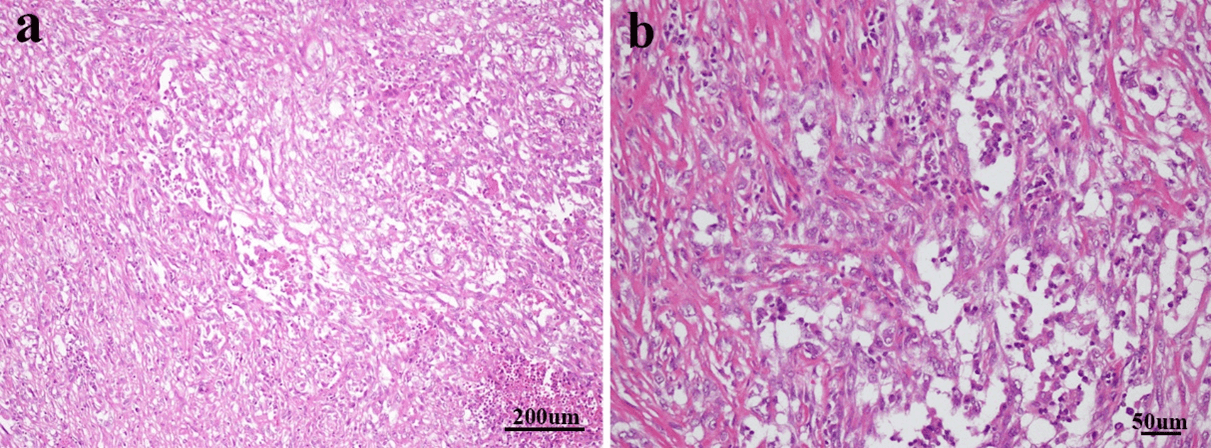
Figure 2: a) The cells of sarcomatous area are spindled and patchy in distribution. Necrosis could be seen in lower right side (stain, hematoxylin and eosin; magnification, x100). b) Some of sarcomatoid cells were epithelioid, rich in cytoplasm, staining weakly, vacuole nucleus and nucleoli could be seen (stain, hematoxylin and eosin; magnification, x200).
Grossly, the mass of renal pelvis and renal calices sarcomatoid carcinoma is usually large (diameter ≥ 4 cm), and it may be described as solid and papillary and the transverse section was white and gray [2, 4-6]. The structure characteristics in this case are identical with this general description. While the mass in this case also infiltrated to sinus renalis, renal parenchyma and perinephric fat. The tumor consists of high grade sarcomatoid tumor cells. Histological examination can also find tumor cells from epithelium origin and other abnormal cells, which indicating the nature of mixed tumor of this disease [1, 2, 4, 6, 9]. The malignant epithelial cells include transitional cell carcinoma cells, squamous carcinoma cell, adenocarcinoma cell. etc. The real sarcoma cells (osteosarcoma, chondrosarcoma, rhabdomyosarcoma or leiomyosarcoma) were absent in this disease. There is a transitional area between the tissue from epithelium origin and sarcomatoid tissue. Cytoplasm of the tumor cells is eosinophil.
The tumor cells have increased size of the nucleus; increased chromatin content; abnormal chromatism distribution. The tumor usually possesses large number of mitoses, especially producing atypical ones [1, 4, 6]. The tumor cells in this case were spindled and patchy in distribution. Necrosis could be seen. Some tumor cells were epithelioid, rich in cytoplasm, staining weakly, vacuole nucleus and nucleoli could be seen (Figures 2a & 2b).
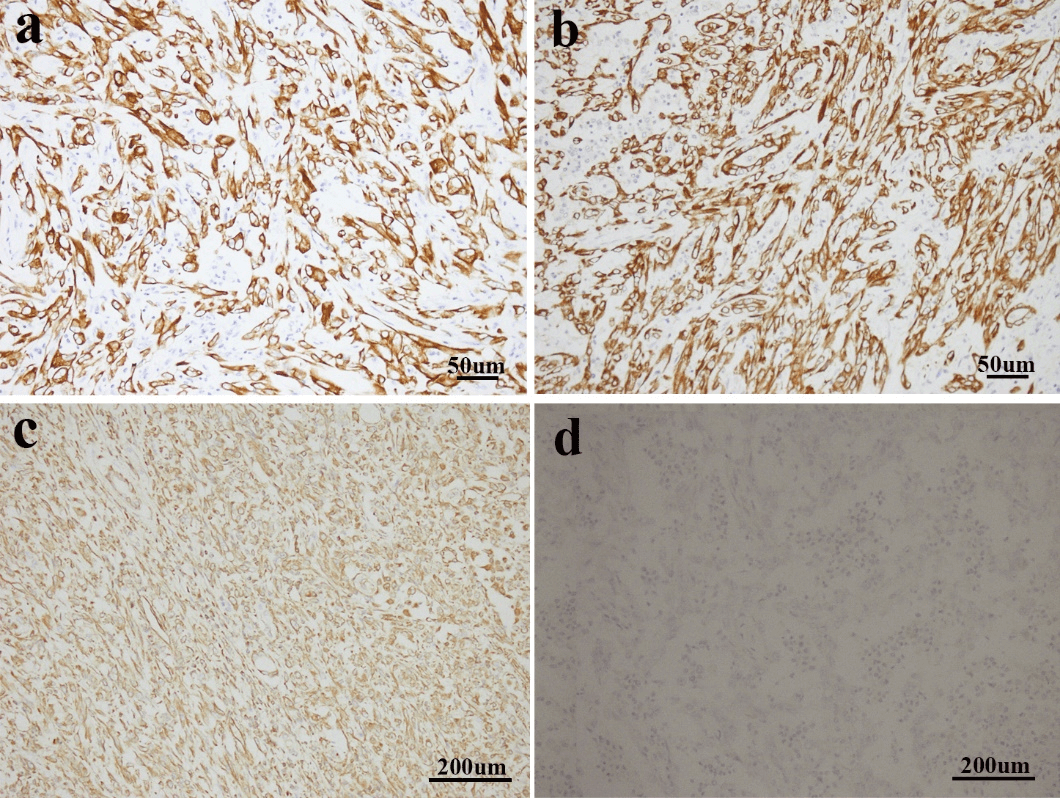
Figure 3: a & b) Sarcomatoid cells demonstrated positive expression of CK7 and CK-pan (immunostaining; magnification, x200). c) Vimentin was strongly expressed in the sarcomatoid area (immunostaining; magnification, x100). d) Sarcomatoid cells showed negative expression of P63 (immunostaining; magnification, x100).
Immunohistochemistry (IHC) test is crucial for the diagnosis of renal pelvis and renal calices sarcomatoid carcinoma. Typically, both CK and vimentin stainings are positive. The tumor cells origin from epithelium express CK and EMA, while tumor cells from mesenchymal tissues express vimentin. Both CK and vimentin positivity may indicate the process of metaplasia from epithelium to mesenchymal tissues in the tumor. Desmin, muscle specific actin and S-100 protein are usually not expressed [2, 3, 6, 10]. The IHC results for this patient agreed with the general features above mentioned (Figures 3a-3c). Combined with IHC with morphology results, the definite diagnosis could be confirmed. P63 is homologous with p53. The positive P63 may be associated with the higher grading/higher staging and the sarcomatoid nature of the tumor [3, 11]. We performed P63 staining for this case and it is negatively stained (Figure 3d).
Differential diagnosis also relies on the combination of IHC and morphology analyses. In clinical practice, renal pelvis and renal calices sarcomatoid carcinoma should be distinguished from the following tumors: 1) Sarcoma, epithelium IHC biomarkers are negative for sarcoma. In addition, cytodesma and intermediate filament are absent in sarcoma. 2) Renal sarcomatoid carcinoma, this is a high-grade malignant tumor in renal parenchyma. The differences between this disease and renal pelvis and renal calices sarcomatoid carcinoma are the gross structure and tumor cell origins. Renal pelvis and renal calices sarcomatoid carcinoma are usually papillary, and transitional carcinoma cells could be found in area of sarcomatoid carcinoma [4, 12]. 3) Carcinosarcoma, this contains both carcinoma and sarcoma in its tumor mass. Pathologists can find osteosarcoma, chondrosarcoma and rhabdomyosarcoma, while IHC evidence in epithelium differentiation lacks in carcinosarcoma. Sarcomatoid carcinoma mainly consist of epithelium tumor cells. Both CK and Vimentin are expressed in sarcomatoid carcinoma [2, 3, 5]. The differential diagnosis between two diseases have no impact on the intervention to the patients. They both indicate quick progression and poor prognosis and the clinical manifestations are similar [4].
There are no standard treatment guidelines for this disease. Most reported cases underwent nephroureterectomy, while almost no chemotherapy or radiotherapy were performed on these patients [2, 7]. Previous study proposed that surgery should be avoided for the tumor staging higher than T4, because these patients may not benefit from surgery [13]. EGFR expression in urethra sarcomatoid carcinoma is upregulated, which indicates that targeting therapy could be a potential treatment option for renal pelvis and renal calices sarcomatoid carcinoma [7]. Furthermore, the prognosis of this disease is poor. Most died in the first two years after diagnosis and long-term survival is scarce [2, 3, 7, 14]. Renal pelvis and renal calices sarcomatoid carcinoma respond poorly to chemotherapy or radiotherapy, and this assistant treatment has little effect on overall survival benefit [2, 6, 7, 13, 14]. Previous studies demonstrated that pathological stage has been associated with prognosis [13].
Conclusion
Renal pelvis and renal calices sarcomatoid carcinoma is rare in clinical practice. It is very difficult to make an early diagnosis due to the lack of specific clinical manifestations. Definite diagnosis relies on the combination of IHC and morphological analyses. There are no standard treatment guidelines for this disease. Most reported cases underwent nephroureterectomy. The prognosis is associated with pathological staging. More data are needed to make evidence-based treatment decisions.
Abbreviations
IHC: Immunohistochemistry
CK: Cytokeratin
EMA: Epithelial Membrane Antigen
SMA: α-Smooth Muscle Actin
EGFR: Epidermal Growth Factor Receptor
Consent
Patient has provided informed consent for publication of the case.
Article Info
Article Type
Case ReportPublication history
Received: Fri 18, Sep 2020Accepted: Thu 01, Oct 2020
Published: Wed 07, Oct 2020
Copyright
© 2023 Di Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.05.01
Author Info
Qiliang Yin Shijie Lan Fengming Jiang Di Wu
Corresponding Author
Di WuTumor Center of JiLin University No.1 Hospital, Changchun, China
Figures & Tables
References
- Canacci AM, MacLennan GT (2006) Sarcomatoid Urothelial Carcinoma of the Renal Pelvis. J Urol 175: 1906. [Crossref]
- Lopez Beltran A, Escudero AL, Cavazzana AO, Spagnoli LG, Vicioso Recio L (1996) Sarcomatoid transitional cell carcinoma of the renal pelvis. A report of five cases with clinical, pathological, immunohistochemical and DNA ploidy analysis. Pathol Res Pract 192: 1218-1224. [Crossref]
- Xiquan Tian, Jiyu Zhao, Yue Wang, Nianzeng Xing (2014) Sarcomatoid carcinoma of the renal pelvis: A case report. Oncol Lett 8: 1208-1210. [Crossref]
- Sekido Y, Satoh F, Usui Y, Tsutsumi Y (2000) Sarcomatoid carcinoma of the renal pelvis: A case report. Pathol Int 50: 562-567. [Crossref]
- Hisataki T, Takahashi A, Taguchi K, Shimizu T, Suzuki K, et al. (2001) Sarcomatoid transitional cell carcinoma originating from a duplicated renal pelvis. Int J Urol 8: 704-706. [Crossref]
- Acikalin MF, Kabukcuoglu S, Can C (2005) Sarcomatoid carcinoma of the renal pelvis with giant cell tumor-like features: Case report with immunohistochemical findings. Int J Urol 2005, 12: 199-203. [Crossref]
- Wang X, MacLennan GT, Zhang S, Montironi R, Lopez Beltran A et al. (2009) Sarcomatoid carcinoma of the upper urinary tract: clinical outcome and molecular characterization. Human Pathol 40: 211-217. [Crossref]
- Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ et al. (2018) The SCARE 2018 statement: Updating consensus Surgical Case REport (SCARE) guidelines. Int J Surg 60: 132-136. [Crossref]
- Shimasaki N, Inoue K, Nishigawa H, Kuroda N, Shuin T (2005) Combined small cell carcinoma and sarcomatoid squamous cell carcinoma in the renal pelvis. Int J Urol 12: 686-689. [Crossref]
- Masue N, Hasegawa Y, Moriyama Y, Ikeda Y, Gotoh T et al. (2007) Spontaneous disappearance of multiple lung metastases after nephroureterectomy from sarcomatoid carcinoma of the renal pelvis: a case report. Int J Urol 14: 75-78. [Crossref]
- Vermeulen P, Hoekx L, Colpaert C, Wyndaele JJ, Van Marck E (2000) Biphasic sarcomatoid carcinoma (carcinosarcoma) of the renal pelvis with heterologous chondrogenic differentiation. Virchows Arch 437: 194-197. [Crossref]
- Delahunt B (1999) Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology 31: 185-190. [Crossref]
- Terada T (2013) Multiple cytokeratin-negative malignant tumors composed only of rhabdoid cells in the renal pelvis: a sarcomatoid urothelial carcinoma? 6: 724-728. [Crossref]
- Thiel DD, Igel TC, Wu KJ (2006) Sarcomatoid carcinoma of transitional cell origin confined to renal pelvis. Urology 67: 622.e9-622.e11. [Crossref]
