Relationship Between Single Nucleotide Polymorphisms and the Toxicy and Side Effects of Paclitaxel and Platinum-Based Chemotherapy in Patients with Malignant Tumors
A B S T R A C T
Objective: Investigating the relationship between single nucleotide polymorphisms (SNPs) and the toxic and adverse effects of paclitaxel and platinum-based chemotherapy in patients with malignant tumors, to provide recommendations for individualized treatment.
Methods: Determinate 17 patients with malignant tumor DNA site and analysis.
Results: 1. All 17 selected specimens’ fluorouracil related genes 18DPYD*2A(476002G>A)GG type、153DPYD*13(1679T>G)TT type 154DPYD(2846A>T)TT type, and the synthesis of DPYD enzyme activity. 21GSTP1(313A>G) polymorphism site mutation rate was 25.0%(4 cases), 29XRCC1(16323944T>C) polymorphism site mutation rate was 90.9%(10 cases), 62ABCB1(3435C>T) polymorphism site mutation rate was 52.9%(9 cases), and 68MTHFR(677C>T) polymorphism site mutation rate was 50.0%(8 cases).
2. Fluorouracil related genes 18DPYD*2A(476002G>A)GG type, 153DPYD*13(1679T>G)TT type, 154DPYD(2846A>T)TT type ,and the synthesis DPYD enzyme activity is normal.
3. Paclitaxel related genes 62ABCB1(3435T>C) CC type has a lower incidence of hematotoxicity and neurotoxicity, than CT type and TT type. 13ABCB1(2677T>G)GG type has a higher rate of drug resistance than TT type. 14CYP1B1*3(C>G)CC type has a higher progression-free survival. Platinum-related genes 21GSTP1(313A>G)AA is homozygous wild type and has a higher incidence of hematotoxicity than GA type. 29XRCC1(1196T>C)CC is homozygous mutant and has a higher risk of serious neutropenia than CT type. 62ABCB1 (3435T>C)CC is homozygous mutant and has a higher risk of lymphatic metastasis than TC type and TT type. 68MTHFR(677C>T)TT type is homozygous mutant and has a higher mucosal toxicity and toxic and side effects than CT type and CC type.
Conclusion: Single nucleotide polymorphism is related to the toxic and side effects of chemotherapy,the detection of SNP to predict the toxicity risk of drug users can be an important reference index to guide clinical individualized treatment.
Keywords
Single nucleotide polymorphisms(SNPs), malignant tumor, paclitaxel, platinum, chemotherapy, toxic and side effect
Introduction
The lasted data from the World Health Organization (WHO) shows that malignant tumor has been the second leading cause of death worldwide, killing 8.8 million people in 2015. Affected by population growth and aging population, the number of malignant tumor in developing countries is on the increase. 60% of the world's cases have occurred in Africa, Asia and central and South America, and 70% of cancer deaths around the world [1]. At present, surgery, radiotherapy and chemotherapy are still the main methods of tumor treatment and chemotherapy plays an important role in the treatment of many cancers. However, chemotherapy kills tumor cells as well as normal ones, and it may also cause gastrointestinal dysfunction, bone marrow suppression, renal toxicity, liver toxicity, blood toxicity, neurotoxicity and other side effects, thus greatly reduced the effectiveness of chemotherapy and survival quality of patients. The reaction and toxicity of chemotherapeutic drugs are not only related to the age, gender and interactions between drugs, but also related to the expression level of proteins or enzymes involved in drug metabolism. Single nucleotide polymorphisms (SNPs) mainly refers to DNA sequence polymorphism caused by mutation of single nucleotide at the genome level. As the third generation of genetic markers SNPS have been widely used in gene mapping, correlation analysis, population genetics and drug research and so on [2]. It is the most common form of human heritable variation. In recent years, the correlation between SNPs and chemotherapeutic drug reaction and toxicity has been increasing. This study takes common chemotherapeutic drugs as an example, to analyze the relationship between SNPs and fluorouracil, platinum derivatives and paclitaxel.
Data and methods
I Sample collection
All 17 patients with malignant tumors are inpatients in Zhongda Hospital Southeast University. Number them in sequence (1~17). Among them, there are 12 males and 5 females, aged from 31 to 83, and the median age is 60. There are 10 cases of gastric malignant tumors, 1 case of malignant transformation of parotid mixed tumors, 1 case of hypopharyngeal carcinoma, 1 case of pancreatic malignant tumors, 1 case of esophageal cancer, 1 case of peritoneal malignant tumors and 1 case of cervical squamous cell carcinomas. Each patient underwent a comprehensive physical examination and laboratory examination before chemotherapy,including blood routine, liver function test, kidney function test, electrocardiogram, etc, to assess chemotherapy tolerance. Patients aged > 85 with severe heart, lung and brain disease, severe liver and kidney dysfunction are not included in the group.
II Selection of DNA detection sites
All selected specimens are tested for 18DPYD*2A(476002G>A), 153DPYD*13(1679T>G),154DPYD(2846A>T),13ABCB1(2677T>G), 14CYP1B1*3(G>C), and 62ABCB1(3435C>T). 16 selected specimens are tested for 21GSTP1(313A>G)、68MTHFR(677C>T). 11 selected specimens are tested for 29XRCC1(16323944T>C). (The leading number represents the site).
III Sampling and testing methods
3ml venous blood which should be stored in the 4℃ or -20℃ environment,and the storage time should not exceed 24h. The collected clinical samples should be numbered (year, month, day, patient number) and mixed.A number of 1.5ml centrifuge tubes were taken and numbered, which was consistent with that on the anticoagulant tubes. 1mL NH4Cl pretreatment solution was added to the 1.5ml centrifuge tube. 200ul of clinical whole blood was mixed and added into the corresponding numbered 1 NH4Cl pretreatment fluid centrifugal tube. After the blood sample was mixed with NH4Cl, the liquid color gradually changed to a clear red.Centrifuge at room temperature 3000rpm (or 500-700g) for 5min to draw the upper transparent red liquid clean and avoid sucking out the white blood cells precipitated at the bottom of the tube. Add 1mL NH4Cl (or normal saline) to completely resuspend the white blood cells, centrifuge at room temperature at 3000rpm (or 500-700g) for 5min and suck up the upper liquid. Pay attention to avoid sucking out the white blood cells at the bottom of the tube. Add 50u detection reagent to the centrifuge tube enriched with white blood cells, and blow and mix repeatedly. Let stand at room temperature for 20-30min, then mix upside down for 2 times. According to the gene loci to be detected, the corresponding stain was taken out and the sample number was marked on the lid of the reagent tube. If the reagent adheres to the tube wall or the upper cover, centrifugation is required to ensure the integrity of the detection system. White blood cell samples treated with 1.5ul were added to the corresponding number of staining agents (the samples should be mixed again).It can be detected after short centrifugation.
Results
I The incidence rate of SNPs
All 17 selected specimens’ fluorouracil related genes 18DPYD*2A(476002G>A)GG type 153DPYD*13(1679T>G)TT type 154DPYD(2846A>T)TT type, and the synthesis of DPYD enzyme activity. These specimens’ data are in (Table 1).
Table 1:
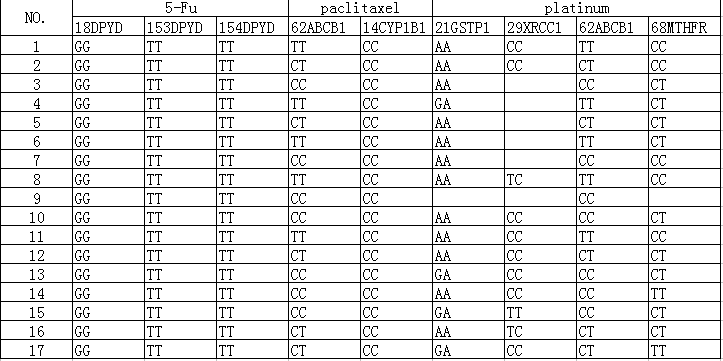
Among them, 21GSTP1(313A>G) polymorphism site mutation rate was 25.0%(4 cases), 29XRCC1(16323944T>C) polymorphism site mutation rate was 90.9%(10 cases), 62ABCB1(3435C>T) polymorphism site mutation rate was 52.9%(9 cases), and 68MTHFR(677C>T) polymorphism site mutation rate was 50.0%(8 cases) (Table 2).
Table 2: Distribution of SNPs in patients with cancer [η/%(n/n)].
|
|
Wild |
Mutation |
|
21GSTP |
75.0(12/16) |
25.0(4/16) |
|
29XRCC1 |
9.1(1/11) |
90.9(10/11) |
|
62ABCB1 |
47.1(8/17) |
52.9(9/17) |
|
68MTHFR |
50.0(8/16) |
50.0(8/16) |
II The relationship between SNP and toxic side effects
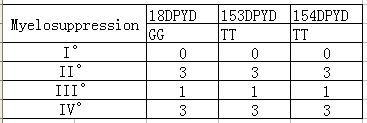
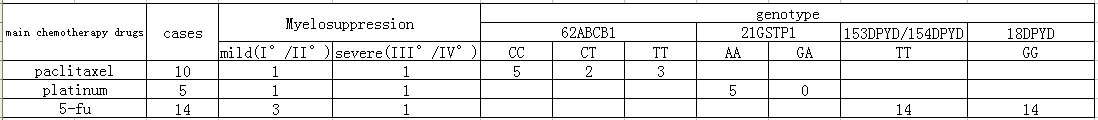
There are 7 cases have various degrees of myelosuppression in 17 specimens, including 0 I ° bone marrow suppression, 3 II ° bone marrow suppressions, 1 III ° bone marrow suppressions and 3 IV ° bone marrow suppressions. By analyzing the genotypes of these patients who had a bone marrow suppression, we can find:
1. Fluorouracil related genes 18DPYD*2A(476002G>A) GG type, 153DPYD*13(1679T>G) TT type, 154DPYD(2846A>T)TT type ,and the synthesis DPYD enzyme activity is normal (Table 3).
Table 3:
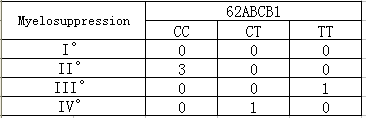
2. Paclitaxel related genes 62ABCB1(3435T>C) CC type has a low incidence of hematotoxicity and neurotoxicity, a high disease control rate and survival rate. CT type has a low incidence of hematotoxicity and neurotoxicity, a medial disease control rate and survival rate. TT type has a high incidence of hematotoxicity and neurotoxicity (Table 4).
Table 4:
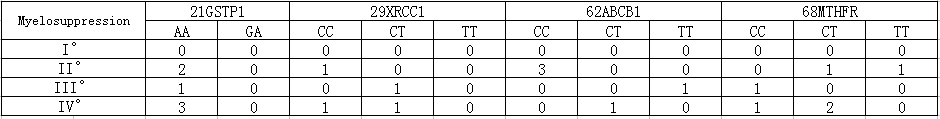
3. Platinum-related genes 21GSTP1(313A>G) AA is homozygous wild type and has a poor response to platinum-based drugs and a high incidence of hematotoxicity. GA type has a medial incidence of hematotoxicity. 29XRCC1(1196T>C)CC is homozygous mutant and has a low drug resistance , a high survival rate and a risk of serious neutropenia. CT type has a medial risk of neutropenia. 62ABCB1 (3435T>C)CC is homozygous mutant, and it has a high risk of lymphatic metastasis and a low rate of survival when it is treated with platinum. TC type is mutant hybrid and has a lower risk of lymphatic metastasis and a higher rate of survival than homozygotic type. TT type has a low risk of lymphatic metastasis and a high survival rate. 68MTHFR(677C>T)TT type is homozygous mutant, and it has a high response to platinum-based drugs and mucosal toxicity and toxic and side effects significantly. CT type is mutant hybrid and has a poor response to platinum-based drugs and side effects lightly. CC type also has a poor response to platinum-based drugs and side effects lightly (Table 5).
Table 5:
4. In these patients, the toxicity risk of drug users can be predicted by pre-detection of SNP and formulating chemotherapy programs which based on these can effectively reduce the occurrence of toxic and side effects (Table 6). These verify that SNP has a relationship with chemotherapeutic drug response and toxicity. And detecting SNP to predict the toxicity risk of drug users will be an important reference index to guide clinical individualized treatment.
Table 6:
Discussion
Fluoropyrimidines (5-fluorouracil and the oral prodrug capecitabine and tegafur) have a prominent role in the treatment of many tumors. DPYD is a rate-limiting enzyme in 5-fu catabolism, effecting on the efficacy and toxicity of 5-fu. DPYD is located in the short arm of chromosome 1, the gene on EXON14 (DPYD*2A) is the most common genetic variation that leads to decreased enzyme activity. About 40% of individuals with low DPYD enzyme activity carry the DPYD*2A allele, and 60% of patients will have grade 4 severe granulocyte reduction after 5-fu treatment. But in patients with normal DPYD enzyme activity, the incidence of severe adverse reactions caused by 5-fu is only 10%. So, monitoring DPYD*2A polymorphism can predict adverse reactions during treatment with 5-fu. There are also some studies showing that detection of DPYD SNP can predict the adverse reactions of 5-fu chemotherapy [3]. The CPIC guide also recommends the use of fluorouracil (5-fu), capecitabine, teflon and other pyrimidine analogues for DPYD polymorphism detection to avoid serious adverse reactions. CYP1B1 protein catalyzes estrogen action. Mutations in CYP1B1*3 sites lead to increased protein-catalyzed estrogens. It may affect the inhibitory effect of docetaxel on microtubules during cell division, leading to decreased response. In the study, it was found that the pCR rate of CYP1B1*3 site mutation (GG type) was significantly lower than that of the wild type (CC type) after taxol treatment.
ABCB1 is a drug transporter, mainly responsible for the transport of a variety of drugs in vivo, and it is involved in paclitaxel drug transport. Several studies have shown that ABCB1 3435 site mutation (TT type) is more likely to cause hematologic toxicity and neurotoxicity than the wild type (CC type), which affects the disease control rate and survival time of patients [4]. The cytotoxic effect of platinoids is mainly to form platinum-dna complex, thus inhibiting DNA replication and transcription. The improvement of DNA damage repair ability is the main reason for the decrease of efficacy of platinum-based chemotherapy, and now the most studied SNPs mainly exists in the nucleotide resection and repair pathway and the base resection and repair pathway. DNA repair is a process involving multiple enzymes and proteins. Once the relevant genes are mutated, the DNA repair ability of the entire genome will be decreased, leading to the occurrence and development of malignant tumors. Studies have shown that XRCC1 (human X - ray staggered complementary repair gene) is widely involved in DNA repair, and Arg194Trp, Arg280His and Arg399Gln have been shown to affect XRCC1,they cause amino acid substitutions in the XRCC1 protein leading to the alteration of its function [5]. Such polymorphisms have been associated to a general increased cancer risk in the full population as a result of impaired capacity of DNA repair, which is correlated to greater tumor aggressiveness, and lower response to platinum derivatives [6]. Detoxification of platinum-based drugs involves the conjugation with reduced glutathione (GSH), a reaction catalyzed by glutathione S-transferase protein 1(GSTP1) [7].
GSTP1 (glutathione s-transferase P1) is one of the members of the supergene family of GSTs, participating in the degradation and excretion of carcinogens. In view of the key role of enzymes involved in either DNA repair or drug metabolism in the pharmacodynamics and pharmacokinetics of platinum salts, several variants of both XRCC1 and GSTP1 have been investigated as potential biomarkers of either response or toxicity [8]. In recent years, a number of pharmacogenomic studies have demonstrated that both efficacy and toxicity of drugs are largely influenced by SNPs, and this is quite important to patients who are suffering from cancers and receiving chemotherapy. Because there is a definite correlation between chemotherapy efficacy/tolerability and survival outcomes, cannot be denied [9]. The detection of SNPs to predict the toxicity risk of drug users will be an important reference index to guide clinical individualized treatment. This paper also verifies the above viewpoints. However, the fact is that there are so many SNPs sites, though people are more and more aware of the relationship between SNP and the response and side effects of chemotherapy drugs, there are still many problems worthy of people to study.
Funding
This work was supported by National Natural Science Foundation of China (NO.81773284).
Article Info
Article Type
Research ArticlePublication history
Received: Wed 18, Sep 2019Accepted: Wed 02, Oct 2019
Published: Mon 14, Oct 2019
Copyright
© 2023 Yi-Qian ZHU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.05.06
Author Info
CHEN Bao-An GUO Nan-Nan WU Yi-Ting LIU Yan-Wen Yi-Qian ZHU
Corresponding Author
Yi-Qian ZHUDepartment of Hematology and Oncology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, China
Figures & Tables

Table 2: Distribution of SNPs in patients with cancer [η/%(n/n)].
|
|
Wild |
Mutation |
|
21GSTP |
75.0(12/16) |
25.0(4/16) |
|
29XRCC1 |
9.1(1/11) |
90.9(10/11) |
|
62ABCB1 |
47.1(8/17) |
52.9(9/17) |
|
68MTHFR |
50.0(8/16) |
50.0(8/16) |




References
- Stewart BW, Wild CP (2014) Word Cancer Report.
- Gao XL, Jing FX, Yang JB, Zhao JL, Single nucleotide polymorphism analysis technique.
- Zhang X, Sun BT, Lu ZX (2011) Relationship between SNP of DPYD and 5-fluorouracil toxicity in colorectal cancer patients 37: 707-711.
- Yang XL, Effects of ABCB1 gene polymorphism on hematological toxicity of docetaxel in breast cancer patients.
- Shen MR, Jones IM, Mohrenweiser H (1998) Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res 58: 604-608. [Crossref]
- Yi L, Xiao-Feng H, Yun-Tao L, Hao L, Ye S et al. (2013) Association between the XRCC1 Arg399Gln polymorphism and risk of cancer: evidence from 297 case-control studies. PLoS One 8: e78071. [Crossref]
- Sharma A, Pandey A, Sharma S, Chatterjee I, Mehrotra R et al. (2014) Genetic polymorphism of glutathione S-transferase P1 (GSTP1) in Delhi population and comparison with other global populations. Meta Gene 20: 134-142. [Crossref]
- Raffaele P, Claudia C, Erica S, Mauro C, Stefania LS et al. (2018) SNPs in predicting clinical efficacy and toxicity of chemotherapy: walking through the quicksand. Oncotarget 9: 25355-25382. [Crossref]
- Evans WE, McLeod HL (2003) Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med 348: 538-549. [Crossref]
