Regenerative Effect of Intraovarian Injection of Activated Autologous Platelet Rich Plasma: Serum Anti-Mullerian Hormone Levels Measured Among Poor-Prognosis In Vitro Fertilization Patients
Regenerative Effect of Intraovarian Injection of Activated Autologous Platelet Rich Plasma: Serum Anti-Mullerian Hormone Levels Measured Among Poor-Prognosis In Vitro Fertilization Patients
A B S T R A C T
This registered, prospective clinical trial assessed serum anti-Mullerian hormone (AMH) patterns after treatment with activated platelet rich plasma (PRP). Patients with low ovarian reserve and/or at least 1 prior failed in vitro fertilization (IVF) cycle (n=182) received PRP injected into ovarian tissue under ultrasound guidance. Pretreatment AMH, BMI and platelet (PLT) concentration were recorded and serum AMH, follicle stimulating hormone, and estradiol were then measured at 2-week intervals for up to three months. Mean±SD patient age was 45.4±6.1yrs. Improved serum AMH was observed in 51 patients (28%) with median increase of 167% [95%CI 91; 280] after treatment; mean interval to maximum AMH increase was 4 weeks (range 2-10 weeks). Improved post-treatment AMH was not limited to younger patients; when stratified by age (<42 vs. ≥42yrs), significant AMH improvements were seen in both groups after treatment (p=0.03 and 0.009, respectively). Among responders, mean basal PLT count was higher (274K) vs. non-responders (250K); p<0.001. This is the first clinical trial to describe an intraovarian PRP technique for low reserve and finds the treatment safe and associated with significant increases in serum AMH for some patients, usually within four weeks. The substantially different pre-treatment PLT concentrations measured across PRP response groups warrants further investigation. Additional research can characterize ovarian response better, optimize PRP protocols, and collect outcomes data from those who subsequently undergo IVF with autologous oocytes.
Keywords
Reproductive aging, platelet rich plasma, ovary, endogenous hormones
Introduction
Platelet rich plasma (PRP) comprises many soluble mediators which coordinate cellular repair after tissue injury [1]. Closely linked to inflammatory signaling, PRP is also involved in tissue regeneration, cell proliferation, extracellular matrix remodeling, apoptosis, differentiation, and angiogenesis [2]. Platelets play an important role in the local tissue repair response following ovarian epithelial microtrauma after ovulation, and likely contribute to overall organ function as well [3]. Recognizing the need to explore the action of platelets and their derivatives on the adult human ovary, a prospective clinical trial was launched to address this issue. Of note, even before patient enrollment closed, intraovarian application of autologous PRP was occasionally noted to alter ovarian reserve, permit retrieval of oocytes, culture and transfer of embryos. Moreover, reproductive outcomes after similar PRP techniques described in two early reports were favorable, underscoring the urgent need for further research [4, 5]. Thus, the current descriptive, prospective clinical trial aimed to improve the understanding of this treatment and offer insights as to why it might be useful for poor-prognosis fertility patients.
Table 1. Measured changes in selected ovarian reserve markers following intraovarian injection of autologous activated platelet rich plasma (PRP).
|
Patient age (yrs) |
N |
BMI |
Baseline/pre-treatment |
|
post-PRP |
tMAX1 |
p2 |
||||
|
|
|
|
d3 FSH |
d3 E2 |
AMH |
|
d3 FSH |
d3 E2 |
AMH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<42 |
48 |
23.6±5.3 |
43.1±5.9 |
91.5±15.8 |
0.21±0.5 |
|
54.3±7.3 |
53.7±8.1 |
0.32±0.08 |
4.5±0.44 |
0.030 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≥42 |
134 |
23.3±4.9 |
56.1±4.1 |
63.4±6.2 |
0.17±0.03 |
|
68.4±4.7 |
53.4±7.1 |
0.21±0.04 |
4.6±0.24 |
0.009 |
BMI: body mass index (m/kg2), day #3 FSH: follicle stimulating hormone (mIU/mL), day #3 E2: estradiol (pg/mL), AMH: serum antimüllerian hormone (ng/mL). 1 mean interval between PRP injection and maximal AMH response (weeks post-PRP); 2 comparison of pre- vs. post- PRP serum AMH, by paired t-test.
Results
Data were collected prospectively on patients who completed ovarian PRP and necessary monitoring (n=182). Entry criteria was disclosed on the registered clinical trials website and all patients underwent prescreening by telephone interview; none were blocked from participating. Mean ±SD age was 45.4±6.1yrs. Pre-PRP mean±SD body mass index (BMI) for patients was 24.5±0.34kg/m2. While serum FSH, E2, and AMH were measured before and during the clinical trial, the latter was considered the primary marker of ovarian reserve, given its relative consistency throughout the menstrual cycle and preferred utility for amenorrhea. Consistent with a poor-prognosis designation, as expected no study participant had pre-treatment serum AMH >1.0ng/mL (Table 1). All patients tolerated the ovarian PRP injection procedure well and there were no complications.
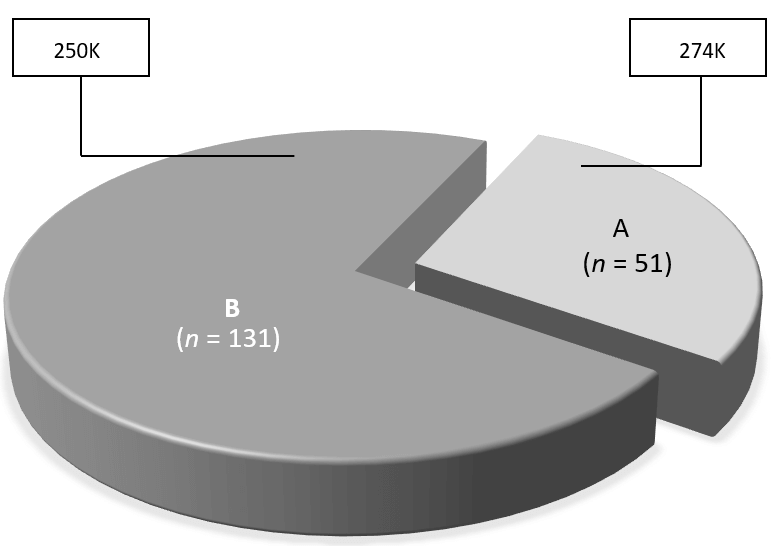
Figure 1: Observed changes in serum AMH following intraovarian PRP injection among registered study participants (n=182), showing distribution of responders (A) and non-responders (B) with associated mean pre-treatment platelet concentrations measured in each group (box).
Category A (i.e., those with improved post-PRP serum AMH response, n=51) accounted for 28% [95% CI 21.6; 35.1] of the study group, while 131 patients (72%) showed no change or reduced serum AMH after treatment as ‘Category B’ (Figure 1). Baseline BMI was not significantly different among these patients (mean BMI A vs. B, 24.7 [95% CI 23.6; 25.7] vs. 24.4 [95% CI 23.5; 25.2]; p=0.71). Of note, the observed maximum BMI among Category A and B patients was 33.3 and 45.5kg/m2, respectively. In contrast, assessment of baseline platelet (PLT) concentrations for Category A and B patients revealed a significant difference, such that “good responder” (Category A) patients had higher mean platelet count than those in Category B (mean PLT for A vs. B = 274K [95% CI 250; 298] vs. 250K [95% CI 239; 261], p<0.001. Not surprisingly, most participants in this study were over age 42yrs (n=134). As noted in (Table 1), when patients were stratified by age at study entry (<42 vs. ≥42yrs), both groups experienced significant improvements in serum AMH after ovarian PRP injection (p=0.03 and 0.009, respectively).
As serum FSH is commonly used to estimate ovarian reserve, measured post-PRP serum AMH responses were analyzed as a function of basal (pre-treatment) FSH. \At study entry, FSH was significantly different (p<0.001) between Category A vs. B patients, with mean FSH noted at 22.1 [95% CI 13.8; 30.4] and 64.1 [95% CI 56.3; 72], respectively. All patients in this study consented for both ovaries to be injected with autologous PRP, but bilateral dosing was not possible for some women. Unilateral ovarian injection most often occurred due to poor visibility via transvaginal ultrasound secondary to body habitus [5]. Nevertheless, placement of autologous PRP in just one ovary did not impact the pattern of serum AMH recorded after PRP, as unilateral ovarian administration of PRP was observed similarly in 25.5% and 35.1% of Category A and B patients, respectively (p=0.21).
Discussion
The apparently unstoppable consequences of advancing female age remain vexing for fertility patients and IVF providers alike. As a physiologic process, perimenopause and menopause are typified by highly variable symptoms impacting not just reproductive outcome, but also general productivity and overall quality of life [6]. This ovarian senescence is measured by an agonal decline in female fertility, gradually decaying ovarian reserve, and a therapeutic requiem which usually concludes with a familiar coda-use of oocytes donated from a younger woman [7-9]. This final act is accepted by some IVF patients, but egg donation is not applauded by others. These data suggest a new intervention to alter this cadence.
Application of autologous PRP recently emerged as an alternate ending to egg donation, and this pioneering composition to “rejuvenate” adult ovarian function foreshadowed two publications describing ovarian tissue treatment with autologous PRP specifically as a prelude to IVF [10]. Using this approach, we described four poor-prognosis IVF patients (mean age 42yrs) who had been consigned to donor oocyte treatment-all produced blastocysts from their own eggs after ovarian PRP and one has since undergone thaw, transfer, and healthy term delivery [5]. Six months later, experts in Greece reported on three poor-responder IVF patients (mean age 38yrs) with similar “revolutionary” outcomes [4]. Of note, or at least one patient with a history of producing consecutive aneuploid embryos intraovarian injection of platelet-derived growth factors before IVF achieved what appeared to be a qualitative “ploidy rescue”, with subsequent healthy term delivery [11]. Parallel to these reports, the first intraovarian PRP clinical trial explored this procedure in a much larger sample. Here, results are presented with insights regarding strengths and weaknesses of this technique.
This first ovarian PRP clinical trial offers a mixed picture. At the outset, our work is best considered a feasibility study to improve serum AMH. A ligand of ovarian origin, AMH reflects aggregate granulosa compartment activity and therefore oocyte production potential during follicular recruitment and IVF [12]. Although consensus on AMH sample preparation techniques may not be universal, the test is nevertheless applied routinely to exclude patients for IVF when the result falls below some (usually arbitrary) threshold [13]. Given the role of AMH as a gatekeeper in egg donation its use as a benchmark for this research seemed reasonable [14, 15]. We observed significant increases in AMH after intraovarian PRP in >25% of patients. While improved serum AMH was noted as early as few weeks after treatment, more research is required to know why measurable gains in AMH sometimes required extra time to develop (and sometimes did not develop at all). In addition, the apparent similarity in post-treatment AMH response as a function of bilateral vs. unilateral injection raises new and intriguing questions about growth factor communication and transmission to non-contiguous ovarian tissues.
Improved ovarian reserve was previously correlated inversely with BMI after autologous intraovarian PRP injection, but this was not validated by larger sampling here [5]. In contrast, the current investigation sharpens the understanding of platelet (PLT) dynamics. The broad normal range of PLT coupled with larger IVF patient numbers in the present study clarified how PLT concentration might affect subsequent serum AMH response. Specifically, a significantly higher mean baseline PLT was measured in PRP-responders vs. non-responders (p<0.001). This is the first research implicating PLT count as a parameter likely to predict AMH response to intraovarian PRP injection. Indeed, discovering that PLT concentration relates to AMH response after intraovarian PRP treatment militates against the theory that simply sticking a needle into the ovary (sham injection or “ovarian acupuncture”) is sufficient to evoke a meaningful AMH change. More work is needed to ascertain what other aspects of PLT function may influence ovarian tissue response to this treatment.
What might explain the treatment effects observed in these poor-prognosis IVF patients who received intraovarian PRP? Similarities exist between wound healing and ovarian tissue repair following capsule rupture at ovulation, and molecular signaling events which might be necessary to reverse the effects of reproductive aging seem congruent with changes occurring in tissue injury responses elsewhere [16]. Growth factors derived from PRP include multiple regulatory proteins which attach to cell membrane receptors and mediate important chemical messages. Via this interaction, they enable inter- and intracellular signaling pathways directing cell growth, proliferation, and differentiation. Unlike hormones, growth factors from PRP show quite circumscribed action, working only in very close proximity to their release site. Such local effects include mitogenesis, angiogenesis, chemotaxis, and formation of the extracellular matrix and even controlling release of other growth factors. For example, platelet-derived growth factor (PDGF) was originally identified in platelets and in serum as a mitogen for fibroblasts, smooth muscle cells (SMC), and glia cells in culture. PDGF has since expanded to a family of dimers of at least four gene products, whose biological actions are mediated through two receptor tyrosine kinases.
In the adult ovary, signaling derivatives of activated platelets might act upon specific populations of progenitor cells to generate different cell types with distinct functions in a variety of developmental processes. Given the wide scope of action, it is plausible that PRP elements could provide requisite signal(s) needed to induce precursor or stem cell differentiation into mature oocytes [16]. This inference is supported by prior work showing that rescue from developmental arrest depends on PDGF (and other platelet-derived mediators like IGF-1) where these cytokines trigger DNA synthesis and cell-cycle specific proto-oncogenes fos and myc with entry into mitosis within 24h [17-19]. PDGF research has also led to an understanding of how cells detect a gradient of attractant, advancing towards it [20]. Guidance of cell migration during ovarian development suggests a mechanistic overlap with axon pathfinding and cell migration [21]. This role of PDGF in guiding cell migration already has been studied directly in vivo [22]. The migration of somatic (border) cells in Drosophila was chosen as model for directional migration in a genetically tractable system, where cells were seen to delaminate from the anterior follicular epithelium and move toward the oocyte. Upon arrival at the egg, they migrate a short distance dorsally toward the germinal vesicle, a PDGF-modulated process crucial for female fertility [23].
There are important limitations to this work which warrant discussion. Although elevated AMH after this PRP protocol is welcomed by older patients with previously low reserve aspiring to begin an IVF cycle, this is not the same thing as a healthy term livebirth or even successful blastocyst development. But IVF cannot begin—at least for now—without eggs, and serum AMH is a proxy marker for retrieving them [24]. These descriptive findings would have been strengthened by comparisons with a matched control group. How ovarian PRP patients perform later in ovulation induction and IVF is now only just beginning to be studied [4, 5, 16]. While non-reproductive use of intraovarian PRP was outside the scope of this clinical trial, estradiol and testosterone are both ovarian modulators of female sexual and neurobehavioral response and appear to be substantially enhanced after PRP treatment [25-28].Familiarity with PRP used in other clinical settings has prompted awareness and wider acceptance of this technology by IVF patients. Progress in clinical IVF practice is achieved by incremental gains, and we consider these data on PRP as a first report on how it may help women with borderline or absent ovarian reserve attain their personal reproductive goals.
Methods
After institutional review board approval, this prospective registered clinical trial (NCT03178695) opened for menopausal and peri-menopausal women in April 2017. Those eligible to enroll had at least one ovary, infertility of >1yr duration, at least one prior failed (or canceled) IVF cycle, or amenorrhea for at least three months (some study patients were considered so unsuited for fertility treatment that they were never permitted to attempt IVF using native oocytes elsewhere). All procedures were performed by staff affiliated to an independent clinical unit in Southern California (Gen 5 Fertility Center; San Diego). Written informed consent was obtained from all study participants. Exclusion criteria included ongoing pregnancy, current or previous IgA deficiency, ovarian insufficiency secondary to sex chromosome etiology, prior major lower abdominal surgery resulting in pelvic adhesions, anticoagulant use for which plasma infusion is contraindicated, psychiatric disorder precluding study participation (including active substance abuse or dependence), ongoing malignancy, or chronic pelvic pain [29].
I Sample Preparation
Approximately 8-10 mL whole blood was collected by peripheral venipuncture from each patient using a 21G butterfly catheter affixed via vacutainer to negative pressure receiving tubes (RegenLab; Mont-sur-Lausanne, CH). Samples were immediately labeled and placed in room-temperature centrifuge set to 1500g x5 min [30]. Processed blood was then fractionated, and erythrocytes were trapped beneath while lower density components settled atop the separator gel. Less than 3 mL of supernatant (corresponding to relatively platelet-poor plasma fraction) was then aspirated off the top of each column before recapping the vial for gentle tube inversion/resuspension, as per supplier instructions.
II Substrate Activation and Ovarian Injection Technique
PRP activation was achieved with calcium gluconate similar to previous protocols [30, 31]. In summary, 10cc syringes were used to divide activated PRP samples into two equal portions and maintained at room temperature, then attached to a 35cm single lumen 19G needle assembly (Rocket Medical; Washington, UK). The injection apparatus was modified for office PRP administration by bypassing the Falcon tube collection port to allow direct injection into ovarian stroma under transvaginal ultrasound guidance. The ovaries were aligned with the needle guide to avoid intervening vascular or other structures and the needle was quickly advanced without rotation deep into the central ovary. Once tip placement was confirmed, activated substrate was slowly introduced as the needle was withdrawn across the previously traversed ovarian cortex. The final ~1mL of sample was deposited just under the ovarian capsule. After injection, careful ultrasound assessment of the pelvis was performed to assure vascular integrity and absence of free pelvic fluid. No sedation or anesthesia was used for any ovarian PRP injection; for all study patients this was completed in less than 10min. At the conclusion of the ovarian injection procedure, each patient was asked to remain at rest in supine position x15 min; vital signs were rechecked before home discharge.
III Assessment of Post-PRP Ovarian Response
All patients had testing for serum AMH, estradiol (E2) and FSH at approximately two-week intervals after ovarian PRP. This was compared to baseline levels obtained within two weeks prior to treatment. To simplify analysis, patient responses to intraovarian PRP in this population were classified as follows: increase in serum AMH vs. baseline level (Category A) or no change/decrease in serum AMH vs. baseline level (Category B). Increases and decreases were assessed at any higher or lower result measured following intraovarian PRP dosing, without respect to duration of any observed change. ‘No change’ was defined as patient serum AMH level variances of ≤5% after intraovarian PRP injection.
Because AMH measurement variance can be introduced by differing reagent dilutions or non-standardized assay platforms all testing was performed on uniform, consistent immunoassay equipment for each patient [13]. To reduce observer measurement variation across multiple centers and personnel performing transvaginal ultrasound, the antral follicle count was regarded as too unreliable and therefore not used as a marker of post-treatment ovarian response.
IV Statistics
Chi-square test was used for equality of proportions. Maximum likelihood estimation (MLE) was used to determine proportions with 95% confidence interval to assure ≥95% coverage when data distributions were approximately normal. For highly skewed values, median and confidence intervals were determined by non-parametric sign test. P-values <0.05 were considered statistically significant. Dispersion of patient age was shown by catplot functions with Seaborn V9.0 visualization library in Python V3.6.5 [32].
Author Contributions
ESS was principal investigator and developed the manuscript, NSR and JLP coordinated patient care and data entry, XL assisted with research design and statistical assessments, SHW provided editorial input and supervised the overall project. All authors read and approved the final product.
Conflicts of interest
ESS holds a provisional U.S. patent for process & treatment using ovarian platelet rich plasma. NSR, JLP, XL and SHW have no conflicts to disclose.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 10, Feb 2020Accepted: Sat 22, Feb 2020
Published: Wed 18, Mar 2020
Copyright
© 2023 E. Scott Sills. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2020.01.02
Author Info
E. Scott Sills J. L. Petersen N. S. Rickers Samuel H. Wood Xiang Li
Corresponding Author
E. Scott SillsGen 5 Fertility Center; San Diego, California, USA
Figures & Tables
Table 1. Measured changes in selected ovarian reserve markers following intraovarian injection of autologous activated platelet rich plasma (PRP).
|
Patient age (yrs) |
N |
BMI |
Baseline/pre-treatment |
|
post-PRP |
tMAX1 |
p2 |
||||
|
|
|
|
d3 FSH |
d3 E2 |
AMH |
|
d3 FSH |
d3 E2 |
AMH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<42 |
48 |
23.6±5.3 |
43.1±5.9 |
91.5±15.8 |
0.21±0.5 |
|
54.3±7.3 |
53.7±8.1 |
0.32±0.08 |
4.5±0.44 |
0.030 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
≥42 |
134 |
23.3±4.9 |
56.1±4.1 |
63.4±6.2 |
0.17±0.03 |
|
68.4±4.7 |
53.4±7.1 |
0.21±0.04 |
4.6±0.24 |
0.009 |
BMI: body mass index (m/kg2), day #3 FSH: follicle stimulating hormone (mIU/mL), day #3 E2: estradiol (pg/mL), AMH: serum antimüllerian hormone (ng/mL). 1 mean interval between PRP injection and maximal AMH response (weeks post-PRP); 2 comparison of pre- vs. post- PRP serum AMH, by paired t-test.
References
- Nurden AT (2011) Platelets, inflammation and tissue regeneration. Thromb Haemost 105: S13-S33. [Crossref]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453: 314-321. [Crossref]
- Lacci KM, Dardik A (2010) Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med 83: 1-9. [Crossref]
- Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pantou A et al. (2018) A Case Series on Platelet-Rich Plasma Revolutionary Management of Poor Responder Patients. Gynecol Obstet Invest 84: 99-106.
- Sills ES, Rickers NS, Li X (2018) First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol 34: 756-760. [Crossref]
- Greening J (2017) Menopause transition: effects on women’s economic participation. HM Government Equalities Office [United Kingdom].
- Sills ES, Alper MM, Walsh AP (2009) Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol 146 :30-36. [Crossref]
- Rosenwaks Z, Navot D, Veeck L, Liu HC, Steingold K et al. (1988) Oocyte donation. The Norfolk Program. Ann N Y Acad Sci 541: 728-741. [Crossref]
- Sauer MV, Paulson RJ, Lobo RA (1992) Reversing the natural decline in human fertility. An extended clinical trial of oocyte donation to women of advanced reproductive age. JAMA 268: 1275-1279. [Crossref]
- Pantos K, Nitsos N, Kokkali G (2016) Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. In: Abstracts, ESHRE 32nd Annual Meeting [Helsinki] 3-6 July, 2016. Hum Reprod i301.
- Sills ES, Rickers NS, Svid C, Rickers JM, Wood SH (2019) Normalized ploidy following 20 consecutive blastocysts with chromosomal error: Healthy 46, XY pregnancy with IVF after intraovarian injection of autologous enriched platelet-derived growth factors. Int J Mol Cell Med 8: 84-89.
- Sills ES, Collins GS, Brady AC, Walsh DJ, Marron KD et al. (2011) Bivariate analysis of basal serum anti-Müllerian hormone measurements and human blastocyst development after IVF. Reprod Biol Endocrinol 9: 153. [Crossref]
- Marron KD, Sills ES, Cummins PL (2016) Impact of pre-mixing AMH serum samples with standard assay buffer: Ovarian reserve estimations and implications for clinical IVF providers. J Reprod Endocrinol Infertil 1: 10.
- Martínez F, Clua E, Carreras O, Tur R, Rodríguez I et al. (2013) Is AMH useful to reduce low ovarian response to GnRH antagonist protocol in oocyte donors? Gynecol Endocrinol 29: 754-757. [Crossref]
- Aghssa MM, Tarafdari AM, Tehraninejad ES, Ezzati M, Bagheri M et al. (2015) Optimal cutoff value of basal anti-mullerian hormone in Iranian infertile women for prediction of ovarian hyper-stimulation syndrome and poor response to stimulation. Reprod Health 12: 85. [Crossref]
- Sills ES, Wood SH (2019) Autologous activated platelet rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep 39: BSR20190805. [Crossref]
- Müller R, Bravo R, Burckhardt J, Curran T (1984) Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature 312: 716-720. [Crossref]
- Pardee AB (1989) G1 events and regulation of cell proliferation. Science 246: 603-608. [Crossref]
- Larson RC, Ignotz GG, Currie WB (1992) Platelet derived growth factor (PDGF) stimulates development of bovine embryos during the fourth cell cycle. Development 115: 821-826. [Crossref]
- Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84: 359-369. [Crossref]
- Wu YC, Horvitz HR (1998) C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392: 501-504. [Crossref]
- Duchek P, Somogyi K, Jékely G, Beccari S, Rørth P (2001) Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17-26. [Crossref]
- Montell DJ, Rørth P, Spradling AC (1992) slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71: 51-62. [Crossref]
- Lee Y, Kim TH, Park JK, Eum JH, Lee HJ et al. (2018) Predictive value of antral follicle count and serum anti-Müllerian hormone: Which is better for live birth prediction in patients aged over 40 with their first IVF treatment? Eur J Obstet Gynecol Reprod Biol 221: 151-155. [Crossref]
- Dhanuka I, Simon JA (2015) Flibanserin for the treatment of hypoactive sexual desire disorder in premenopausal women. Expert Opin Pharmacother 16: 2523-2529. [Crossref]
- Cappelletti M, Wallen K (2016) Increasing women's sexual desire: The comparative effectiveness of estrogens and androgens. Horm Behav 78: 178-193. [Crossref]
- Fantasia HC (2016) Flibanserin and Female Sexual Desire. Nurs Womens Health 20: 309-314. [Crossref]
- Sills ES, Li X, Rickers NS, Wood SH, Palermo GD (2019) Metabolic and neurobehavioral response following intraovarian administration of autologous activated platelet rich plasma: First qualitative data. Neuro Endocrinol Lett 39: 427-433. [Crossref]
- U.S. National Library of Medicine. Autologous platelet-rich plasma (PRP) infusions and biomarkers of ovarian rejuvenation and ageing mitigation (NCT03178695); March 7, 2017.
- Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K (2014) Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through a one-year period. J Cutan Aesthet Surg 7: 213-219. [Crossref]
- Vahabi S, Yadegari Z, Mohammad Rahimi H (2017) Comparison of the effect of activated or non-activated PRP in various concentrations on osteoblast and fibroblast cell line proliferation. Cell Tissue Bank 18: 347-353. [Crossref]
- Waskom ML, Kiani R (2018) Decision making through integration of sensory evidence at prolonged timescales. Curr Biol 28: 3850-3856. [Crossref]
