Prognostic Factors for Cervical Cancer in FIGO-Stages IA-IIB in a 10-Year Period in the Region of Uppsala, Sweden: Population Cohort Study
A B S T R A C T
Background: The incidence of cervical cancer in Sweden decreased to 8/100,000 in the year 2011 but has from 2014 increased to 11/100,000. The noted increase was, mainly observed in FIGO-stages IA-IB, where patients usually are asymptomatic and detected in screening.
Materials and Methods: The study population consisted of 253 patients with cervical carcinoma in FIGO-stages IA-IIB. The patients were referred to the Department of Gynecology at the Akademiska University Hospital in Uppsala for decision of treatment from 2008 to 2017.
Results: Clinical and pathological features of cervical carcinoma were compared according to the subtypes: squamous cell carcinoma (n=150), adenocarcinoma (n=74) and adeno-squamous cancer (n=29). Other rare histological types (n = 6) were excluded. Finally, 207 (82%) out of the 253 referred patients, had primary surgical treatment and 29 (14%) patients had additional oncological treatment. The remaining 46 patients (18%) received primary oncological treatment. In the present study 45 (17.8%) developed recurrent disease. Prognostic factors for disease-free survival in Cox-regression analysis were stage (IA versus IIB) (p = 0.000) and histopathologic subtype (p = 0.009). In a survival analysis the disease-free survival differed between the histological subtypes; 62 % for squamous cell cancer in cervix, 48 % for adenocarcinoma, and 32 % for adeno-squamous cancer (Chi-square = 6,221; p = 0,045)). Logistic regression analysis including treatment details, showed no other predictive factors for recurrent disease.
Conclusion: The results from this study suggest that prognostic factors for disease-free survival are FIGO-stage (IA –IIB) and histopathology, but not tumor grade, age or choice of treatment.
Keywords
Cervical cancer, prognostic factors, treatment, survival
Introduction
The cervical cancer incidence in Sweden decreased from 24/100,000 in 1965 to 8/100,000 in 2011, but it has increased to 11/100,000 from 2014 [1, 2]. The increase appears to correlate to screening history were the incidence in Sweden, as in many western countries, decreased markedly after the introduction of cytological screening in the 1960s [2]. However, from 2014 the incidence of cervical cancer in Sweden had an about 20% increase, which is corresponding to approximately 100 extra annual new cases compared to the previous period. A previous report suggests that the overall increase is associated with an increased cancer risk in women aged below 65 in early FIGO stages (IA-IB), adequately screened with normal cytological and especially in some counties (laboratories) in Sweden results [2, 3].
Carcinoma of the uterine cervix is globally the second most common tumor in women. More than 80% of cervical cancer cases occur in developing countries with 483 000 estimated new cases and 274 000 deaths yearly [4]. Furthermore, GLOBOCAN 2018 database has published ASR (age standardized rates per 100.000) and cumulative risks to age 75 by sex (female / male) and cancer site worldwide 2018, that estimated number of new cervix cancer cases were 569.8 with ASR =13.1 and Cumulative Risk = 1.36 [5].
The FIGO classification is still the most commonly used staging system for female genital cancers. However, according to the latest FIGO staging guidelines, examination under anaesthesia including cystoscopy, are no longer mandatory and radiological tumor volume as well as parametrial invasion should be recorded separately [6]. In the Nordic countries, the annual incidence of new cervical cancer cases was 1390 in 2011-2015 [7]. Pre-treatment diagnostic imaging has reported to affect the FIGO stage in more than half of the Nordic centers and the pre-treatment planning at multi-disciplinary team meetings incorporate findings from imaging examinations such as ultrasound, magnetic resonance imaging (MRI) in combination with PET-CT [6].
In England, a retrospective population-based observational study using register data of 11.951 women diagnosed with cervical cancer between 2006 and 2010 was undertaken to explore how the different morphological subtypes affected survival [8]. Age-standardized survival rates by morphological subtype were, presented alongside with excess mortality modelling accounting for the impact of demographic, diagnostic and tumor factors. The three main morphological subtypes (squamous cell carcinoma, adenocarcinoma and adeno-squamous carcinoma) in that study had similar one-year net survival rates of approximately 85%, and there were no differences at five years with adjusted survival rates of 55-65%.
However, some earlier studies have reported worse survival for patients with adeno-squamous cervical cancer with advanced stage, but not early stage (IB-IIB) disease [9]. Furthermore, in a study from China including 8751 patients in FIGO stages I-IV all treated with definitive radiotherapy, both cancer-specific and overall survival was worse for patients with adenocarcinoma and adeno-squamous carcinoma compared with squamous cell carcinoma [10]. In this retrospective study, we will investigate how the three main morphological subtypes (squamous cell carcinoma, adenocarcinoma and adeno-squamous carcinoma) have impact on recurrence and survival.
Materials and Methods
Patients with biopsy verified cervical carcinoma were referred from hospitals within the Uppsala Medical Region to the regional referral center in the Department of Gynecology at the Akademiska University Hospital in Uppsala for decision of treatment during a 10-year period from January 1, 2008 to December 31, 2017 (Table 1). Samples were collected, in compliance with the Helsinki Declaration and used in accordance with the Swedish Biobank Legislation [11]. The Ethical Review Act was approved by the Uppsala Ethical Review Board, decision (Dnr.2018 / 322) [12].
Table 1: Patient´s
characteristics (n=259) for all patients in FIGO-stages IA-IIB. The study
included 253 patients after six patients with other histology were, excluded
(clear cell tumors (n = 2), neuroendocrine tumors (n = 2) and spino-celluler
tumors (n = 2)) which, are presented in this table, but not included in in this
work.
|
Patient´s
characteristics (n=259) |
n |
% |
|
Age
(median) |
45.9
years (range 22-83 years) |
|
|
FIGO-stage |
259 |
|
|
IA |
45 |
(17.4) |
|
IB |
162 |
(62.5) |
|
IIA |
14 |
(5.4) |
|
IIB |
38 |
(14.7) |
|
Histopathology |
|
|
|
Squamous
cell carcinoma |
150 |
(57.9) |
|
Adenocarcinoma |
74 |
(28.6) |
|
Adeno-squamous |
29 |
(11.2) |
|
Other* |
6 |
(4.6) |
|
Tumor
grade |
|
|
|
G1 |
58 |
(21.4) |
|
G2 |
77 |
(28.4) |
|
G3 |
89 |
(32.8) |
|
Ungraded |
35 |
(12.9) |
|
Type
of Operative treatment |
210 |
|
|
Trachelectomy |
27 |
(13.1) |
|
Total
hysterectomy |
30 |
(14.3) |
|
Radical
hysterectomy |
153 |
(72.6) |
|
Primary
oncological treatment |
49 |
|
|
Radio-chemotherapy |
40 |
(81.6) |
|
Primary
radiotherapy |
9 |
(18.4) |
|
Postoperative
oncological treatment |
29 |
|
|
Radio-chemotherapy |
27 |
(93.1) |
|
Radiotherapy |
2 |
(6.9) |
Other*
tumors of other histology (clear cell tumors (n = 2), neuroendocrine tumors (n
= 2) and spino-cellular tumors (n = 2) are presented in this table, but not
included in this work.
Totally 253 patients with the histological diagnosis of squamous cell cancer, adenocarcinoma and adeno-squamous cancer in cervix in FIGO stages IA-IIB were included in this study, but other rare histological types (n = 6) presented in the (Table 1), were excluded from statistical analysis. The records and histopathologic reports of the 253 patients were retrospectively, reviewed and the variables studied are shown in the (Tables 1, 2, 3 & 4). Clinical examination and FIGO staging by an expert gynaecologist and a gynaecologic oncologist were undertaken. Histology was determined at the time of diagnosis at a central tumor board conference before final decision of treatment (primary oncologic treatment or operation) with results of CT and MRI presented. However, the final histopathological diagnosis was determined post-operatively at a new central tumor board conference for decision of eventual postoperative treatment.
Table 2: Clinical
and pathological features of cervical carcinoma, compared after the subtypes squamous
cell carcinoma, adenocarcinoma and adeno-squamous cancer, (n=253).
|
Histology |
Squamous
cell carcinoma |
Adeno-carcinoma |
Adeno-
squamous carcinoma |
P value |
|
|
No
(%) |
No
(%) |
No
(%) |
|
|
|
150
(%) |
74
(%) |
29(%) |
|
|
Age |
46.8
years |
44.3
years |
44.2
years |
|
|
(t-test) |
|
|
|
0.369 |
|
FIGO-stage |
|
|
|
|
|
IA |
33
(22) |
9
(12) |
3
(10) |
|
|
IB |
86 (57) |
50 (68) |
22 (76) |
|
|
IIA |
7 (5) |
5
(7) |
2 (7) |
|
|
IIB |
24 (16) |
10 (13) |
2 (7) |
|
|
(chi-2 test) |
|
|
|
0.282 |
|
Tumor
grade |
|
|
|
|
|
G1 |
17
(30) |
38
(67) |
2
(3) |
|
|
G2 |
47
(63) |
20
(26) |
9
(12) |
|
|
G3 |
59
(69) |
10
(12) |
16
(19) |
|
|
Ungraded |
27
(77) |
6
(17) |
2
(6) |
|
|
(chi-2 test) |
|
|
|
0.000 |
|
Recurrent disease |
|
|
|
|
|
Without |
120
(80) |
66
(89) |
22
(76) |
|
|
With |
30
(20) |
8
(11) |
7
(24) |
|
|
(chi-2 test) |
|
|
|
0.152 |
Table 3: Treatment modality compared
after the subtypes squamous cell carcinoma, adenocarcinoma and adeno-squamous
cancer, (n=253).
|
Histopathology |
Squamous
cell carcinoma |
Adeno-carcinoma |
Adeno-
squam. Ca |
P value |
|
|
No
(%) |
No
(%) |
No
(%) |
|
|
|
150
(%) |
74
(%) |
29(%) |
|
|
Operative
treatment |
|
|
|
|
|
Total
(n = 207) |
|
|
|
|
|
Radical |
|
|
|
|
|
hysterectomy |
73
(48.7 %) |
54
(36.0 %) |
23
(15.3 %) |
|
|
(n
= 150) |
|
|
|
|
|
Total |
|
|
|
|
|
hysterectomy |
25
(83 % ) |
5
(17 %) |
0 |
|
|
(n
= 30) |
|
|
|
|
|
Trachelectomy |
21
(77.8 %) |
5
(18.5 %) |
1
(0.37%) |
|
|
(n
= 27) |
|
|
|
|
|
|
119 |
64 |
24 |
|
|
(chi-2 test) |
|
|
|
0.003 |
|
Post-operative
oncological treatment (n = 29) |
|
|
|
|
|
Radio-chemotherapy |
14
(52 %) |
5
(18 %) |
8
(30 %) |
|
|
(n=27) |
|
|
|
|
|
Radiotherapy
(only) |
1
(50 %) |
|
1
(50 %) |
|
|
(n=2) |
|
|
|
|
|
(chi-2 test) |
|
|
|
0.775 |
|
Primary
oncological treatment (n = 46) |
|
|
|
|
|
Radio-chemotherapy |
23
(62 %) |
10
(30 %) |
4
(8 %) |
|
|
Primary
radiotherapy |
8
(89 %) |
0 |
1
(11%) |
|
|
(chi-2 test) |
|
|
|
0.170 |
Table 4A: Cox-regression. Prognostic factors for disease-free survival for the
whole series of patients (n=253).
|
Adjusted results |
||||||||
|
Variable |
HR |
-95% C.I. |
+95% C.I. |
HR |
-95% C.I. |
+95% C.I. |
p-value |
|
|
Age |
1,022 |
1,002 |
1,042 |
1,008 |
0,985 |
1,032 |
0,488 |
|
|
FIGO-stage |
|
|
|
|
|
|
0,000 |
|
|
IA |
0,104 |
0,031 |
0,352 |
0,186 |
0,043 |
0,809 |
0,025 |
|
|
IB |
0,193 |
0,101 |
0,367 |
0,181 |
0,086 |
0,383 |
0,000 |
|
|
IIA |
0,230 |
0,068 |
0,780 |
0,235 |
0,066 |
0,837 |
0,025 |
|
|
IIB |
Reference |
Reference |
||||||
|
Histopathology |
|
|
|
|
|
|
0,009 |
|
|
Adeno-carcinom |
0,307 |
0,114 |
0,826 |
0,190 |
0,060 |
0,601 |
0,005 |
|
|
Squamous cell |
0,422 |
0,188 |
0,950 |
0,310 |
0,130 |
0,739 |
0,008 |
|
|
Adeno-squam. |
Reference |
Reference |
||||||
|
Tumor grade |
|
|
|
|
|
|
0,305 |
|
|
G1 |
6,072 |
0,757 |
48,682 |
6,471 |
0,642 |
65,235 |
0,113 |
|
|
G2 |
8,366 |
1,103 |
63,455 |
5,156 |
0,540 |
49,233 |
0,154 |
|
|
G3 |
8,553 |
1,147 |
63,808 |
3,618 |
0,365 |
35,893 |
0,272 |
|
|
Ungraded |
Reference |
Reference |
||||||
Table 4B: Logistic regression. Predictive
factors for recurrent disease for the whole series of patients (n=253).
|
Variable |
OR |
-95% C.I. |
+95% C.I. |
OR |
-95% C.I. |
+95% C.I. |
p-value |
|
Age |
1,029 |
1,007 |
1,052 |
1,002 |
0,976 |
1,030 |
0,865 |
|
FIGO-stage |
0,150 |
||||||
|
IA |
0,061 |
0,016 |
0,231 |
0,093 |
0,010 |
0,862 |
0,037 |
|
IB |
0,130 |
0,059 |
0,288 |
0,143 |
0,023 |
0,898 |
0,038 |
|
IIA |
0,232 |
0,055 |
0,970 |
0,210 |
0,037 |
1,183 |
0,077 |
|
IIB |
|
|
|
|
|||
|
Histopathology |
0,114 |
||||||
|
adenocarcinom |
0,318 |
0,106 |
0,952 |
0,242 |
0,061 |
0,958 |
0,043 |
|
Squamous cell |
0,656 |
0,265 |
1,626 |
0,626 |
0,228 |
1,715 |
0,362 |
|
Adenosquamous |
|
|
|
||||
|
FIGO-grade |
|
0,630 |
|||||
|
G1 |
2,612 |
0,521 |
13,098 |
3,048 |
0,467 |
19,872 |
0,244 |
|
G2 |
4,459 |
0,969 |
20,517 |
2,439 |
0,410 |
14,505 |
0,327 |
|
G3 |
4,923 |
1,083 |
22,373 |
1,888 |
0,311 |
11,479 |
0,490 |
|
Ungraded |
|||||||
|
Treatment |
0,170 |
||||||
|
Operation only |
0,115 |
0,054 |
0,247 |
0,677 |
0,119 |
3,861 |
0,661 |
|
Op.+ postop. |
0,416 |
0,153 |
1,128 |
1,806 |
0,299 |
10,897 |
0,519 |
|
Prim. onk. treatment.
|
|
Statistical Methods
The Pearson’s Chi-square test was, used for testing proportional differences in univariate analyses. The survival curves were, generated by using the Kaplan-Meier technique and differences between curves were, tested by the Chi-square test. Cox regression analysis was, used to estimate the impact of prognostic factors for disease-free survival. Predictive factors for recurrent disease were, analysed with logistic regression models. All tests were two-sided and the level of statistical significance was p ≤ 0.05. The STATISTICA 13.4 (Stat Soft TM) statistical package was used for the analyses.
Results
Totally, 207 (82%) out of 253 patients included in the study, had primary surgical treatment and the remaining 46 patients (18%) received primary oncological treatment (radio-chemotherapy or radiotherapy). Clinical and pathological features of cervical carcinoma, compared according to the subtypes squamous cell carcinoma (n=150), adenocarcinoma (n=74) and adeno-squamous cancer (n=29) are shown in the (Tables 2 & 3). No association between the three histological subtypes and age (p=0.369), FIGO stage (p=0.282) or recurrent disease (p=0.152) respectively, could be detected.
However, the difference in distribution of tumor grade between the three different histological subtypes was highly significant (p=0.000), where adenocarcinoma usually (67 %) were G1 tumors (well differentiated) compared with squamous cell carcinoma (G1:30 %) and adeno-squamous carcinoma (3 %), respectively. On the contrary, tumors of squamous cell carcinoma most often (69 %) were G3 tumor (low differentiated) compared with adenocarcinoma (12 %) and adeno-squamous carcinoma (19 %). Furthermore, in this study, the ungraded tumors usually were of squamous cell carcinoma histology. Thus, 27 /35 ungraded tumors (77 %) were of squamous cell carcinoma histology compared with six (17 %) of adenocarcinoma histology and two (6 %) of adeno-squamous histology, respectively (Table 2).
Totally, 207 out of the 253 patients in the study had primary surgical treatment and the type of surgery chosen for the three histological types differed (p=0.003) (Table 3). Thus, 73/119 patients (61.3 %) with squamous cell carcinoma were treated with radical hysterectomy, compared with 54/64 patients (84.0 %) with adenocarcinoma tumors and 23/24 patients (96 %) with tumors of adeno-squamous histology. For the ungraded tumors 32/35 patients were diagnosed in stage IA and only 11 /32 (34 %) were operated with radical hysterectomy, 15 (47%) with total hysterectomy and six (18%) patients were treated with trachelectomy. The three remaining patients (two patients in FIGO-stage IIB and one patient in FIGO-stage IIA) all had treatment with primary radio-chemotherapy.
In total, 29/207 (14 %) operated patients had postoperative oncological treatment (Table 3) and the remaining patients had surgical treatment only. There was no difference between the three histological groups according to post-operative treatment with 27/29 (93 %) patients treated with radio-chemotherapy and two (7 %) patients had only radiotherapy.
The indication for post-operative radio-chemotherapy was in four patients limited to pelvic lymph node metastases. For the other 23 patients the reason for postoperative treatment included negative prognostic factors such as size of the tumor, tumor invasion of the parametrium or lymph-vascular space invasion and for 5/23 patients it was a combination with pelvic lymph node metastases.
Furthermore, in patients treated with post-operative radio-chemotherapy or radiotherapy 24/29 (83 %), were in pre-operative stage IB (p=0.000) and three patients were in pre-operative stage IIA. The two resting patients both were in stage IA with tumor of squamous cell carcinoma and adeno-squamous histology, respectively, both had treatment with radiotherapy only (Table 3). Thus, it could be concluded, that post-operative oncological treatment was given to 29 (14.0 %) out of 207 patients after preoperative FIGO-staging with clinical examination in combination with preoperative MRT and CT.
In this study 46 (18.2 %) out of the 253 patients had primary oncological treatment with no difference between the three histological subtypes, squamous cell carcinoma, adenocarcinoma and adeno-squamous cancer (p = 0.172) (Table 3). Thirty-seven (80.4 %) out of the 46 patients were treated with radio-chemotherapy and nine (19.6 %) were treatment with primary radiotherapy. It also was, noted, that 28 (82.3%) patients out of the 37 patients treated with primary radio-chemotherapy were in FIGO-stage II B. However, comparing pre-operative FIGO-stage (IA-IIB) between the group of patients treated with primary radiotherapy (n=9) and the group of 37 patients treated with primary radio-chemotherapy, no differences could be detected.
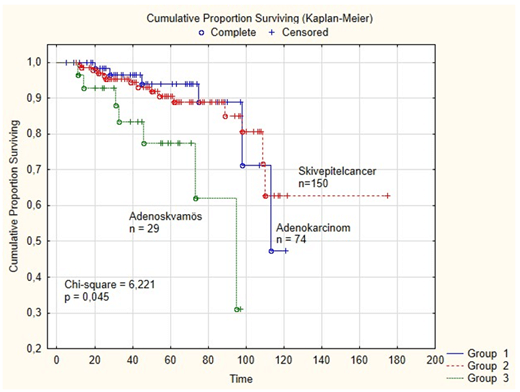
In the present study 45 patients (17.8%), developed recurrent disease. In this study age was not a prognostic factor for survival or predictive factor for recurrent disease. However, in a Cox-regression analysis histopathology (squamous cell cancer, adenocarcinoma or adeno-squamous cancer) was a significant prognostic factor for disease-free survival (p = 0.009) as presented in (Table 4A). Furthermore, in the survival analysis (Figure 1), the 5–year disease-free survival differed between the 3 histopathological sub-groups, (62 % for squamous cell cancer, 48 % for adenocarcinoma and 32 % for adeno-squamous cancer) (Chi-square = 6,221; p = 0,045). No association was found between the FIGO stages (IA-IIB) and the histological subgroups in a univariate analysis (Table 2), but in a Cox-regression analysis (Table 4A), FIGO-stage (IA-IIB) was a highly significant (p = 0.000) prognostic factor for disease-free survival. The tumor grade was not significant prognostic factor for disease-free survival in a Cox-regression analysis (p = 0.305).
Figure 1: The 5–year disease-free survival was different between the 3 histopathological sub-groups, (62 % for squamous cell cancer, 48 % for adenocarcinoma and 32 % for adeno-squamous cancer (Chi-square = 6,221; p = 0,045).
However, in logistic regression analysis (Table 4B) the new variable “treatment” was added to the analysis for predictive factors for recurrent disease (Age, FIGO-stage, Histopathology and FIGO grade), but not any significant predictive factor for recurrent disease could be noted in that analysis. Perhaps, those findings could, be explained by the thesis that every patient received her optimal treatment (operation only, post-operative treatment after her operation or primary oncological treatment).
Discussion
The present study included 253 patients with cervical carcinoma in FIGO-stages IA-IIB, referred to the Department of Gynecology at the Akademiska University Hospital for decision of treatment during the 10-year period, 2008 to 2017.
In the univariate analysis, including clinical and pathological features of cervical carcinoma, no differences for age, FIGO-stage or recurrent disease could, be detected between the three main subtypes, squamous cell carcinoma, adenocarcinoma and adeno-squamous cancer. However, the differences in tumor grade was highly significant between the three different histological groups in a univariate analysis, but in the regression analysis, the tumor grade was not prognostic factor for disease-free survival or predictive factor for recurrent disease. On the contrary, the FIGO-stage (IA-IIB) was a highly significant prognostic factor for disease-free survival, but not a predictive factor for recurrent disease.
Finally, histopathology (adenocarcinoma, squamous cell cancer and adeno-squamous cancer) was a highly significant prognostic factor for disease-free survival, but not a predictive factor for recurrent disease. As mentioned above, the tumor grade differed between the three different histological groups in a univariate analysis, but in a Cox-regression analysis, the tumor grade was not prognostic factor for disease-free survival. Thus, tumors of adenocarcinoma usually were, well differentiated (67 %) compared with squamous cell carcinoma (30 %) and tumors of adeno-squamous carcinoma (3 %), respectively. On the contrary, tumors of squamous cell carcinoma usually were low differentiated (69 %) compared with tumors of adenocarcinoma (12 %) and adeno-squamous carcinoma (19 %), respectively.
However, the tumor grade usually is not a prognostic factor in squamous cervical cancer and not taken into account for patient treatment and guidelines for the management of patients with cervical cancer according to the European Society of Gynecological Oncology (ESGO) [13]. Thus, according to ESGO the major tumor-related prognostic factors are FIGO or TNM stage, including a maximum tumor size, extra-cervical tumor extension and nodal involvement. Furthermore, information about the tumor type and depth of cervical stromal invasion is important and finally, presence or absence of lympho-vascular space involvement and the presence or absence of distant metastases [13].
A retrospective observational study from USA including 31.536 women between 1983 and 2013 was, undertaken to examine the association between tumor grade and survival for women with squamous cervical cancer [14]. Among 4045 women in FIGO stage I disease, who underwent surgical treatment without radiotherapy, both grade 2 tumors (HR=2.54) and the grade 3 tumors (HR=4.48) were independently associated with decreased cancer specific survival compared to grade 1 tumors. Thus, this study suggested that tumor differentiation grade might be a prognostic factor in women with squamous cervical cancer, particularly in early-stage disease. Furthermore, tumor grade is regularly included in histopathology of cervical squamous and adenocarcinoma. However, at present no grading system has achieved universal acceptance and grading of these tumors remains of uncertain value [15].
In this study, the ungraded tumors usually were of squamous cell carcinoma histology (77 %). In a retrospective population-based observational study of 11.951 women with cervical cancer, the excess mortality rates (1-3 years) increased from six months after diagnosis for ungraded tumors and were similar to low-grade tumors [8]. Therefore, it could, be suggested that ungraded tumors were likely to be low-grade tumors. Furthermore, should early invasive cervical carcinoma, which may include the tumors that are too small to grade, not routinely be, graded by pathologists [16].
The strength of this study is that it consists of the complete population of cervical cancer cases in FIGO-stages IA-IIB admitted to one single institution for decision of treatment, with detailed data on tumor characteristics, treatment and follow-up. Totally, 253 out of 259 patients were included in this study when rare histological types have, been excluded. In total, 82% of the study population had primary surgical treatment and 14.0 % had indication for further post-operative oncological treatment. Thus, it could, be speculated that the pre-operative work-up including clinical examination in combination with MRT and CT, had some limitations for correct staging. Since presence of lymph node metastases was the major cause of postoperative oncologic treatment, verification of lymph nodes by PET-CT in selected cases could help minimizing the number of women in need for both surgery and postoperative radiotherapy [6]. The newly introduced technique of sentinel node mapping will probably further decrease the negative consequences of combined therapy. In this study 45 patients (17.8 %) developed recurrent disease and Histopathology (adenocarcinoma, squamous cell cancer and adeno-squamous cancer) was a highly significant (p = 0.009) prognostic factor for disease-free survival in the Cox-regression analysis, but not a predictive factor for recurrent disease in the logistic regression analysis.
Furthermore, in a survival analysis (Figure 1), the disease-free survival differed between the three histological subgroups (62 % for squamous cell cancer, 48 % for adenocarcinoma, and 32 % for adeno-squamous cancer). A retrospective population-based observational study from England using cancer registration data of 11.951 women diagnosed with cervical cancer between 2006 and 2010 was, undertaken to explore how the different morphological subtypes affect survival rates [8]. Age-standardized net survival rates by morphological subtype were, presented alongside with excess mortality modelling accounting for the impact of demographic, diagnostic and tumor factors. The three main morphological subtypes (squamous cell carcinoma, adenocarcinoma and adeno-squamous carcinoma) in that study had similar one-year net survival rates of approximately 85%, but there were no differences at five years amongst the three morphological subtypes with adjusted survival rates of 55-65%.
However, some earlier studies have reported worse survival for patients with adeno-squamous cervix cancer in early stage (IB-IIB) whereas other studies were limited to advanced stage [9, 10]. In a study from China, patients in stage IA2-IIA2 underwent radical surgery 2006- 2014 [17]. Of the 5181 patients, 4510 patients had squamous cancer, 488 adenocarcinoma and 183 adeno-squamous cancer and five-year recurrence-free survival was 85.1%, 83.1% and 72.3% respectively (p < 0.001).
Furthermore, these earlier studies have reported different survival between all the three morphological subtypes (squamous cell carcinoma, adenocarcinoma and adeno-squamous carcinoma), but some other studies have compared prognosis and survival only between two morphological subtypes [9, 10]. In a study from Lei et al. it was, reported, that adeno-squamous carcinoma is a histological type of invasive cervical carcinoma that is composed of a mixture of both malignant glandular and squamous components [18].
However, in some studies, prognosis is compared between two histological types only, adeno-squamous carcinoma and adenocarcinoma of cervix or on the contrary, in a study from USA, the subgroup of squamous cell carcinoma was compared with a subgroup of both adenocarcinoma and adeno-squamous carcinoma together [19-21]. Therefore, it is not optimal to compare prognosis for the three histological subtypes in this work with other studies.
Conclusion
In the present study, FIGO-stage and histopathology (adenocarcinoma, squamous cell cancer and adeno-squamous cancer) were highly significant prognostic factors for disease-free survival. Furthermore, survival analysis, comparing the three histological types, reported worse survival for patients with adeno-squamous cervix cancer compared with adenocarcinoma and squamous cell cancer.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 24, Sep 2022Accepted: Thu 13, Oct 2022
Published: Mon 24, Oct 2022
Copyright
© 2023 Ingiridur Skirnisdottir. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2022.02.03
Author Info
Ingiridur Skirnisdottir Karina Varasteh
Corresponding Author
Ingiridur SkirnisdottirDepartment of Women's and Children's Health, Uppsala University, Sweden
Figures & Tables
Table 1: Patient´s
characteristics (n=259) for all patients in FIGO-stages IA-IIB. The study
included 253 patients after six patients with other histology were, excluded
(clear cell tumors (n = 2), neuroendocrine tumors (n = 2) and spino-celluler
tumors (n = 2)) which, are presented in this table, but not included in in this
work.
|
Patient´s
characteristics (n=259) |
n |
% |
|
Age
(median) |
45.9
years (range 22-83 years) |
|
|
FIGO-stage |
259 |
|
|
IA |
45 |
(17.4) |
|
IB |
162 |
(62.5) |
|
IIA |
14 |
(5.4) |
|
IIB |
38 |
(14.7) |
|
Histopathology |
|
|
|
Squamous
cell carcinoma |
150 |
(57.9) |
|
Adenocarcinoma |
74 |
(28.6) |
|
Adeno-squamous |
29 |
(11.2) |
|
Other* |
6 |
(4.6) |
|
Tumor
grade |
|
|
|
G1 |
58 |
(21.4) |
|
G2 |
77 |
(28.4) |
|
G3 |
89 |
(32.8) |
|
Ungraded |
35 |
(12.9) |
|
Type
of Operative treatment |
210 |
|
|
Trachelectomy |
27 |
(13.1) |
|
Total
hysterectomy |
30 |
(14.3) |
|
Radical
hysterectomy |
153 |
(72.6) |
|
Primary
oncological treatment |
49 |
|
|
Radio-chemotherapy |
40 |
(81.6) |
|
Primary
radiotherapy |
9 |
(18.4) |
|
Postoperative
oncological treatment |
29 |
|
|
Radio-chemotherapy |
27 |
(93.1) |
|
Radiotherapy |
2 |
(6.9) |
Other*
tumors of other histology (clear cell tumors (n = 2), neuroendocrine tumors (n
= 2) and spino-cellular tumors (n = 2) are presented in this table, but not
included in this work.
Table 2: Clinical
and pathological features of cervical carcinoma, compared after the subtypes squamous
cell carcinoma, adenocarcinoma and adeno-squamous cancer, (n=253).
|
Histology |
Squamous
cell carcinoma |
Adeno-carcinoma |
Adeno-
squamous carcinoma |
P value |
|
|
No
(%) |
No
(%) |
No
(%) |
|
|
|
150
(%) |
74
(%) |
29(%) |
|
|
Age |
46.8
years |
44.3
years |
44.2
years |
|
|
(t-test) |
|
|
|
0.369 |
|
FIGO-stage |
|
|
|
|
|
IA |
33
(22) |
9
(12) |
3
(10) |
|
|
IB |
86 (57) |
50 (68) |
22 (76) |
|
|
IIA |
7 (5) |
5
(7) |
2 (7) |
|
|
IIB |
24 (16) |
10 (13) |
2 (7) |
|
|
(chi-2 test) |
|
|
|
0.282 |
|
Tumor
grade |
|
|
|
|
|
G1 |
17
(30) |
38
(67) |
2
(3) |
|
|
G2 |
47
(63) |
20
(26) |
9
(12) |
|
|
G3 |
59
(69) |
10
(12) |
16
(19) |
|
|
Ungraded |
27
(77) |
6
(17) |
2
(6) |
|
|
(chi-2 test) |
|
|
|
0.000 |
|
Recurrent disease |
|
|
|
|
|
Without |
120
(80) |
66
(89) |
22
(76) |
|
|
With |
30
(20) |
8
(11) |
7
(24) |
|
|
(chi-2 test) |
|
|
|
0.152 |
Table 3: Treatment modality compared
after the subtypes squamous cell carcinoma, adenocarcinoma and adeno-squamous
cancer, (n=253).
|
Histopathology |
Squamous
cell carcinoma |
Adeno-carcinoma |
Adeno-
squam. Ca |
P value |
|
|
No
(%) |
No
(%) |
No
(%) |
|
|
|
150
(%) |
74
(%) |
29(%) |
|
|
Operative
treatment |
|
|
|
|
|
Total
(n = 207) |
|
|
|
|
|
Radical |
|
|
|
|
|
hysterectomy |
73
(48.7 %) |
54
(36.0 %) |
23
(15.3 %) |
|
|
(n
= 150) |
|
|
|
|
|
Total |
|
|
|
|
|
hysterectomy |
25
(83 % ) |
5
(17 %) |
0 |
|
|
(n
= 30) |
|
|
|
|
|
Trachelectomy |
21
(77.8 %) |
5
(18.5 %) |
1
(0.37%) |
|
|
(n
= 27) |
|
|
|
|
|
|
119 |
64 |
24 |
|
|
(chi-2 test) |
|
|
|
0.003 |
|
Post-operative
oncological treatment (n = 29) |
|
|
|
|
|
Radio-chemotherapy |
14
(52 %) |
5
(18 %) |
8
(30 %) |
|
|
(n=27) |
|
|
|
|
|
Radiotherapy
(only) |
1
(50 %) |
|
1
(50 %) |
|
|
(n=2) |
|
|
|
|
|
(chi-2 test) |
|
|
|
0.775 |
|
Primary
oncological treatment (n = 46) |
|
|
|
|
|
Radio-chemotherapy |
23
(62 %) |
10
(30 %) |
4
(8 %) |
|
|
Primary
radiotherapy |
8
(89 %) |
0 |
1
(11%) |
|
|
(chi-2 test) |
|
|
|
0.170 |
Table 4A: Cox-regression. Prognostic factors for disease-free survival for the
whole series of patients (n=253).
|
Adjusted results |
||||||||
|
Variable |
HR |
-95% C.I. |
+95% C.I. |
HR |
-95% C.I. |
+95% C.I. |
p-value |
|
|
Age |
1,022 |
1,002 |
1,042 |
1,008 |
0,985 |
1,032 |
0,488 |
|
|
FIGO-stage |
|
|
|
|
|
|
0,000 |
|
|
IA |
0,104 |
0,031 |
0,352 |
0,186 |
0,043 |
0,809 |
0,025 |
|
|
IB |
0,193 |
0,101 |
0,367 |
0,181 |
0,086 |
0,383 |
0,000 |
|
|
IIA |
0,230 |
0,068 |
0,780 |
0,235 |
0,066 |
0,837 |
0,025 |
|
|
IIB |
Reference |
Reference |
||||||
|
Histopathology |
|
|
|
|
|
|
0,009 |
|
|
Adeno-carcinom |
0,307 |
0,114 |
0,826 |
0,190 |
0,060 |
0,601 |
0,005 |
|
|
Squamous cell |
0,422 |
0,188 |
0,950 |
0,310 |
0,130 |
0,739 |
0,008 |
|
|
Adeno-squam. |
Reference |
Reference |
||||||
|
Tumor grade |
|
|
|
|
|
|
0,305 |
|
|
G1 |
6,072 |
0,757 |
48,682 |
6,471 |
0,642 |
65,235 |
0,113 |
|
|
G2 |
8,366 |
1,103 |
63,455 |
5,156 |
0,540 |
49,233 |
0,154 |
|
|
G3 |
8,553 |
1,147 |
63,808 |
3,618 |
0,365 |
35,893 |
0,272 |
|
|
Ungraded |
Reference |
Reference |
||||||
Table 4B: Logistic regression. Predictive
factors for recurrent disease for the whole series of patients (n=253).
|
Variable |
OR |
-95% C.I. |
+95% C.I. |
OR |
-95% C.I. |
+95% C.I. |
p-value |
|
Age |
1,029 |
1,007 |
1,052 |
1,002 |
0,976 |
1,030 |
0,865 |
|
FIGO-stage |
0,150 |
||||||
|
IA |
0,061 |
0,016 |
0,231 |
0,093 |
0,010 |
0,862 |
0,037 |
|
IB |
0,130 |
0,059 |
0,288 |
0,143 |
0,023 |
0,898 |
0,038 |
|
IIA |
0,232 |
0,055 |
0,970 |
0,210 |
0,037 |
1,183 |
0,077 |
|
IIB |
|
|
|
|
|||
|
Histopathology |
0,114 |
||||||
|
adenocarcinom |
0,318 |
0,106 |
0,952 |
0,242 |
0,061 |
0,958 |
0,043 |
|
Squamous cell |
0,656 |
0,265 |
1,626 |
0,626 |
0,228 |
1,715 |
0,362 |
|
Adenosquamous |
|
|
|
||||
|
FIGO-grade |
|
0,630 |
|||||
|
G1 |
2,612 |
0,521 |
13,098 |
3,048 |
0,467 |
19,872 |
0,244 |
|
G2 |
4,459 |
0,969 |
20,517 |
2,439 |
0,410 |
14,505 |
0,327 |
|
G3 |
4,923 |
1,083 |
22,373 |
1,888 |
0,311 |
11,479 |
0,490 |
|
Ungraded |
|||||||
|
Treatment |
0,170 |
||||||
|
Operation only |
0,115 |
0,054 |
0,247 |
0,677 |
0,119 |
3,861 |
0,661 |
|
Op.+ postop. |
0,416 |
0,153 |
1,128 |
1,806 |
0,299 |
10,897 |
0,519 |
|
Prim. onk. treatment.
|
|
References
1. Vaccarella S,
Franceschi S, Engholm G, Lönnberg S, Khan S et al. (2014) 50 years of screening
in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 111: 965-969. [Crossref]
2. Dillner J, Sparén P, Andrae B (2019) Cervical cancer is
increasing in women with normal screeing. Livmoderhalscancer-okar-hos-kvinnor-med-normalt-cellprov/
3. Wang J, Andrae B, Strander B, Sparén P, Dillner J
(2020) Increase
of cervical cancer incidence in Sweden in relation to screening history:
populatiocohort study. Acta Oncol 59: 988-993. [Crossref]
4. Ferlay J,
Soerjomataram I, Ervik M (2013) Cancer Incidence and Mortality Worldwide: IARC
Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research
on Cancer.
5. Ferlay J, Colombet M, Soerjomataram I, Mathers C,
Parkin DM et al. (2019) Estimating
the global cancer incidence and mortality in 2018. GLOBOCAN sources and
methods. Int J Cancer 144: 1941-1953. [Crossref]
6. Fuglsang K, Haldorsen IS, Avall Lundqvist E, Lindahl
G, Roed H et al. (2018) Cervical
cancer Staging. Pretreatment planning, and surgical treatment in the Nordic
Countries. Survey from The Surgical Subcommittee of the Nordic Society of
Gynecological Oncology. Acta Obstet Gynecol Scand 97: 1178-1184. [Crossref]
7. NORDCAN (2018)
Available at: http://www-dep.iarc.fr/NORDCAN/DK/ frame.asp.
8. Emmetta M, Gildeaa C, Nordin A, Hirschowitz L, Poole J
(2018) Cervical
cancer - does the morphological subtype affect survival rates? J Obstet
Gynaecol 38: 548-555. [Crossref]
9. Farley JH, Hickey
KW, Carlson JW, Rose GS, Kost ER et al. (2003) Adeno-squamous Histology
Predicts a Poor Outcome for Patients with Advanced Stage, but not Early-Stage,
Cervical Carcinoma. Cancer 97: 2196-2202. [Crossref]
10. Zhou J, Wu SG, Sun
JY, Li FY, Lin HX et al. (2017) Comparsion of clinical outcomes of squamous
cell carcinoma, adenocarcinoma, and adeno-squamous carcinoma of the uterine
cervix after definitive radiotherapy: a population-based analysis. J Cancer
Res Clin Oncol 143: 115-122. [Crossref]
11. WMA Declaration of
Helsinki- Ethical Principles for Medical Research Involving Human Subjects.
http://www.wma.net/en/30publications/10policies /b3/ and used in accordance
with the Swedish Biobank Legislation and Ethical Review Act.
12. Uppsala Ethical
Review Board, decision (Dnr.2018 / 322).
13. Cibula D, Pötter R,
Planchamp F, Avall Lundqvist E, Fischerova D et al. (2018) The European Society
of Gynaecological Oncology/European Society for Radiotherapy and
Oncology/European Society of Pathology. Guidelines for the Management of
Patients with Cervical Cancer. Int J Gynecol Cancer 28: 641-655. [Crossref]
14. Matsuo K,
Mandelbaum RS, Machida H, Purushotham S, Grubbs BH et al. (2018) Association of
tumor differentiation grade and survival of women with squamous cell carcinoma
of the Uterine cervix. J Gynecol Oncol 29: e91. [Crossref]
15. McCluggage WG
(2018) Towards developing a meaningful grading system for cervical squamous
cell carcinoma. J Pathol Clin Res 4: 81-85. [Crossref]
16. Hirschowitz L, Ganesan,
Singh N (2011) Standards and datasets for reporting cancers. The Royal College
of Pathologists. Dataset for histological reporting of cervical neoplasia (3rd
edition).
17. Cao L, Wen H, Feng
Z, Han X, Wu X (2019) Distinctive clinic-pathologic characteristics, and
prognosis for different histologic subtypes of early cervical cancer. Int J
Gynecol Cancer 29: 1244-1251. [Crossref]
18. Lei J, Andrae B,
Ploner A, Lagheden C, Eklund C et al. (2019) Cervical screening and risk of
adenosquamous and rare histological types of invasive cervical carcinoma:
population based nested case-control study. BMJ 365: l1207. [Crossref]
19. Zhang X, Lv Z, Xu
X, Yin Z, Lou H (2020) Comparison of adenocarcinoma and adenosquamous carcinoma
prognoses in Chinese patients with FIGO stage IB-IIA cervical cancer following
radical surgery. BMC Cancer 20: 664. [Crossref]
20. Yun LJ, Lee C, Kim MA, Kim HS, Chung HH et al. (2014) Prognosis of Adeno-squamous carcinoma compared with adenocarcinoma in uterine cervical cancer. A systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 24: 289-294. [Crossref]
21. Seamon LG, Java JJ, Monk BJ, Penson RT, Brown J et al. (2017) Impact of tumour histology on survival in advanced cervical carcinoma: an NRG Oncology / Gynecologic Oncology Group Study. Br J Cancer 118: 162-170. [Crossref]
