Prognostic Factors for Bile Leak and Long-Term Survival in 23 Patients with Klatskin Type III Tumors
A B S T R A C T
Objective: Surgical resection of hilar cholangiocarcinoma carries significant morbidity and mortality, particularly if postoperative bile leak occurs. Prognostic factors and scoring tools have been described for overall morbidity and mortality but none are specific for postoperative bile leak. In this study, we investigate the prognostic utility of various factors in predicting overall morbidity, mortality and risk of biliary leak in Bismuth Type III tumors with the hopes of developing a scoring tool in future research.
Materials and Methods: A retrospective sample of 23 patients with Bismuth Type III tumors exclusively who underwent surgery between 2010 and 2017 were selected for this study. Demographic, surgical, pathologic and biochemical data were collected from the patients’ medical records.
Results: 11 patients underwent a right hepatectomy for type IIIa tumors and 10 patients underwent a left hepatectomy for type IIIb tumors. 2 patients were lost to follow up and were excluded. R0 resection was achieved in 20 patients. Overall survival at 1, 3 and 5 years was 78.3%, 61.9% and 38.1%, respectively. A BMI >24kg/m2 was associated with a worse prognosis, increased overall morbidity and decreased survival at 1, 3 and 5 years (p<0.05). A preoperative creatinine >0.74 was associated with decreased 5-year survival (p<0.05).
Conclusion: A BMI >24kg/m2 and a preoperative creatinine >0.74 are associated with a poor prognosis in Bismuth Type III Klatskin tumors. Furthermore, Age, sex, preoperative hemoglobin, tumor size, use of CUSA and type IIIb tumors demonstrate a borderline significant association with the occurrence of postoperative bile leak.
Keywords
Klatskin tumor, hilar cholangiocarcinoma, hepatectomy, biliary surgery
Introduction
Hilar cholangiocarcinoma (hCCA), or the Klatskin tumor named eponymously after Gerard Klatskin, arises from the extrahepatic biliary epithelium and accounts for 50-60% of cholangiocarcinoma cases, the second most common primary liver tumor after hepatocellular carcinoma [1]. The anatomic classification proposed by Bismuth and Corlette classifies hCCA into 4 types, with type III, which involves the confluence of the left and right hepatic ducts and extends to either the right (IIIa) or left (IIIb) duct, being the most prevalent accounting for 60-80% of cases [2, 3]. Surgery is the standard of care for hCCA [4].
Achieving an R0 resection with negative longitudinal and radial margins is paramount and is associated with increased overall survival [5]. To achieve R0 resections, major hepatectomies are necessary due to the aggressive biology of the hCCA. These tumors exhibit a high degree of lymphovascular invasion and lymphangitis carcinomatosa up to 2cm away from the tumor and towards both the liver and hepatoduodenal ligament [6, 7]. Moreover, a high degree of liver infiltration is seen owing to its frequent location at the hilar plate [8]. Therefore, for the case of type III tumors, a hepatectomy, right for type IIIa and left for type IIIb, is necessary [4].
Hepatic resection for hCCA carries both high morbidity and mortality. In a recent review, postoperative morbidity ranged between 14-66% and mortality between 0-19% [5]. Both rates are significantly higher than similar resections for causes other than hCCA [5]. In one study, 63% of patients developed complications postoperatively with severe complications (Clavien-Dindo Grade 3 and 4) seen in 36.5% [9]. A 90-day mortality of 10% was also reported [9]. Factors predictive of severe complications were serum creatinine, lymphocyte number, the neutrophil-to-lymphocyte ratio, operative time, serum CA 19-9 levels, and the number of transfused blood products [9]. A cause of significant morbidity and mortality is postoperative bile leak (PBL) which can have an incidence of up to 29% [9]. Bile leaks are more likely to occur in wide surface hepatectomies and high-risk surgical inventions, particularly if the major Glisson’s sheath and hepatic hilum are exposed. Furthermore, classically advanced age, high postoperative white blood cell count, prolonged operating time, and left-sided hepatectomies are predictive of PBL [10-12]. These, however, are not specific to resections for hCCA. In Dumitrascu et al.’s study, serum urea levels and neutrophil-to-lymphocyte ratio were found to predict clinically relevant bile leak on univariate analysis however, multivariate analysis did not reveal any independent risk factor [9].
Currently, survival rates at 1, 3 and 5 years are 82%, 45% and 20% respectively [13]. Many studies have both investigated factors and described preoperative and postoperative scoring tools to optimize patient selection and preoperative preparation to improve overall survival and guide neoadjuvant and adjuvant treatment [14, 15]. Factors such as R0 resection, number of harvested lymph nodes, and score systems utilizing serum biomarkers have been described to predict overall survival while others, which utilize patient characteristics and response to portal vein embolization, have been described to predict postoperative liver failure [16-18]. Again, none are specific for the biliary leak. In our study, we investigate the prognostic factors associated with overall postoperative morbidity and mortality of surgical resection of Bismuth-Corlette type III (BCT-III) tumors and attempt to identify those predictive of PBL with the hopes of creating a prognostic scoring tool to further improve patient outcomes and overall survival.
Materials and Methods
I Patient Selection & Data Collection
A retrospective sample of 23 patients who underwent a hepatectomy with curative intent for BCT-III tumors between 2010 and 2017 at Saint George Hospital University Medical Center, a tertiary referral center in Beirut, Lebanon, was selected. Patients with BCT-III tumors that were unresectable were excluded. Demographic, surgical, pathologic and biochemical information were collected from the patients’ medical records. Data collection was performed using Microsoft Excel “Excel version 1908”. Verbal informed consent was obtained from the patient or his representative when applicable. Institutional review board approval was obtained to conduct this study (Ref: IRB-REC/O/017-19/519).
II Preoperative Preparation
Tumor classification was done using the Bismuth-Corlette classification based on preoperative imaging and investigation. Preoperative imaging consisted primarily of computed tomography (CT) scan. Magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography were done on a need by need basis to help define tumor type, locoregional extent of the disease and to rule out distant metastasis. Resectability was defined as follows [19, 20]:
i. BCT-III Tumors (IIIa or IIIb)
ii. No distant metastasis
iii. No invasion of the main portal vein or both its branches
iv. No contralateral involvement of the hepatic artery or portal vein.
III Surgery and Postoperative Care
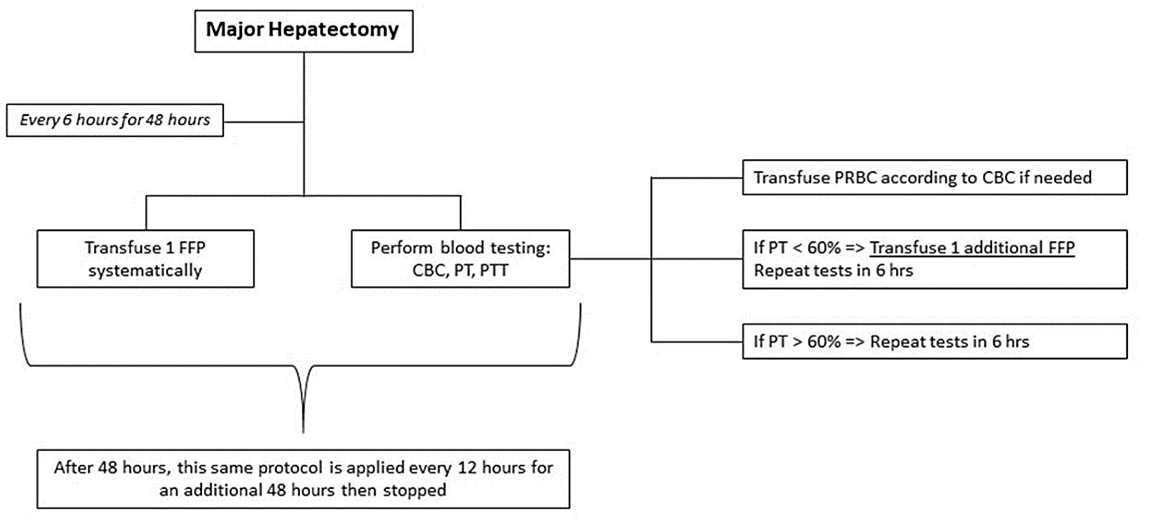
All resections were performed by a single surgeon utilizing the same surgical technique. A right hepatectomy was performed for BCT-IIIa tumors and a left hepatectomy was performed for BCT-IIIb tumors. Two Blake drains were systematically placed at the end of the surgery anterior and posterior to the bilioenteric anastomosis. Patients were observed in the intensive care unit for 48 hours prior to discharge to the floor. Our postoperative care protocol is illustrated in (Figure 1).
Figure 1: Transfusions and Laboratory work-up protocol, adopted at Saint Georges Hospital University Medical Center after Major Hepatectomy (Anatomical Right/ Right Extended or Left/ Left Extended hepatectomy).
FFP: Fresh Frozen Plasma; PRBC: Packed Red Blood Cells; CBC: Complete Blood Count; PT: Prothrombin Time; PTT: Partial thromboplastin time.
IV Statistical Analysis
Descriptive analysis was done using proportions and percentages for categorical variables. Chi-square was used to analyse associations between two independent categorical variables. Student’s t-test was used to analyse associations between independent continuous variables with a normal distribution; otherwise, the Mann-Whitney U test was used. Spearman’s rank-order correlation was used to assess a relationship between two variables. Mortality was defined at 1, 3 and 5 years, and survival analysis was done using the Kaplan-Meier estimator. Statistical significance was defined for a p-value < 0.05. All statistical analysis was performed using SPSS v.24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
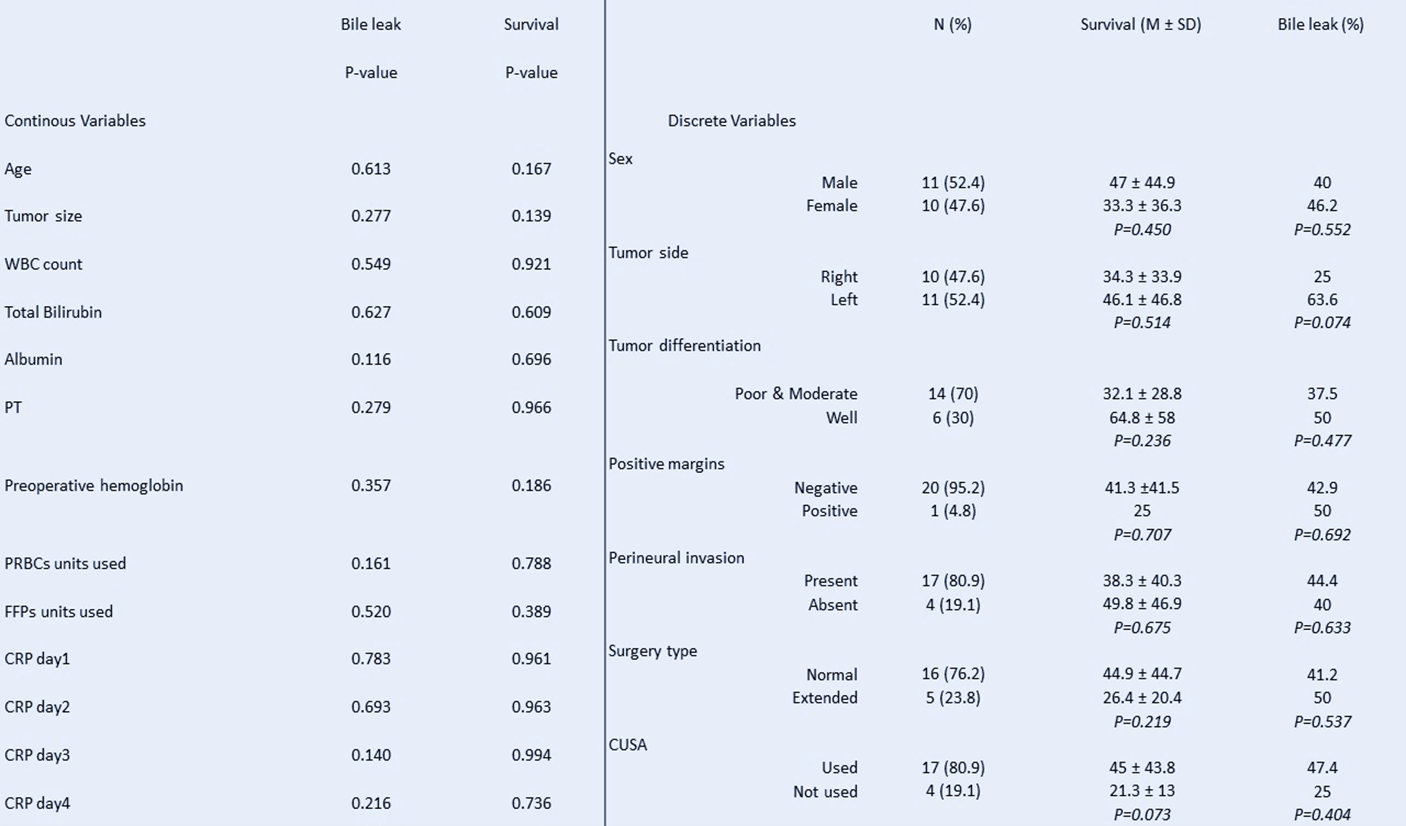
Figure 2: Some of the analysed variables (continuous and discrete), and their effect on Survival and Bile leakage.
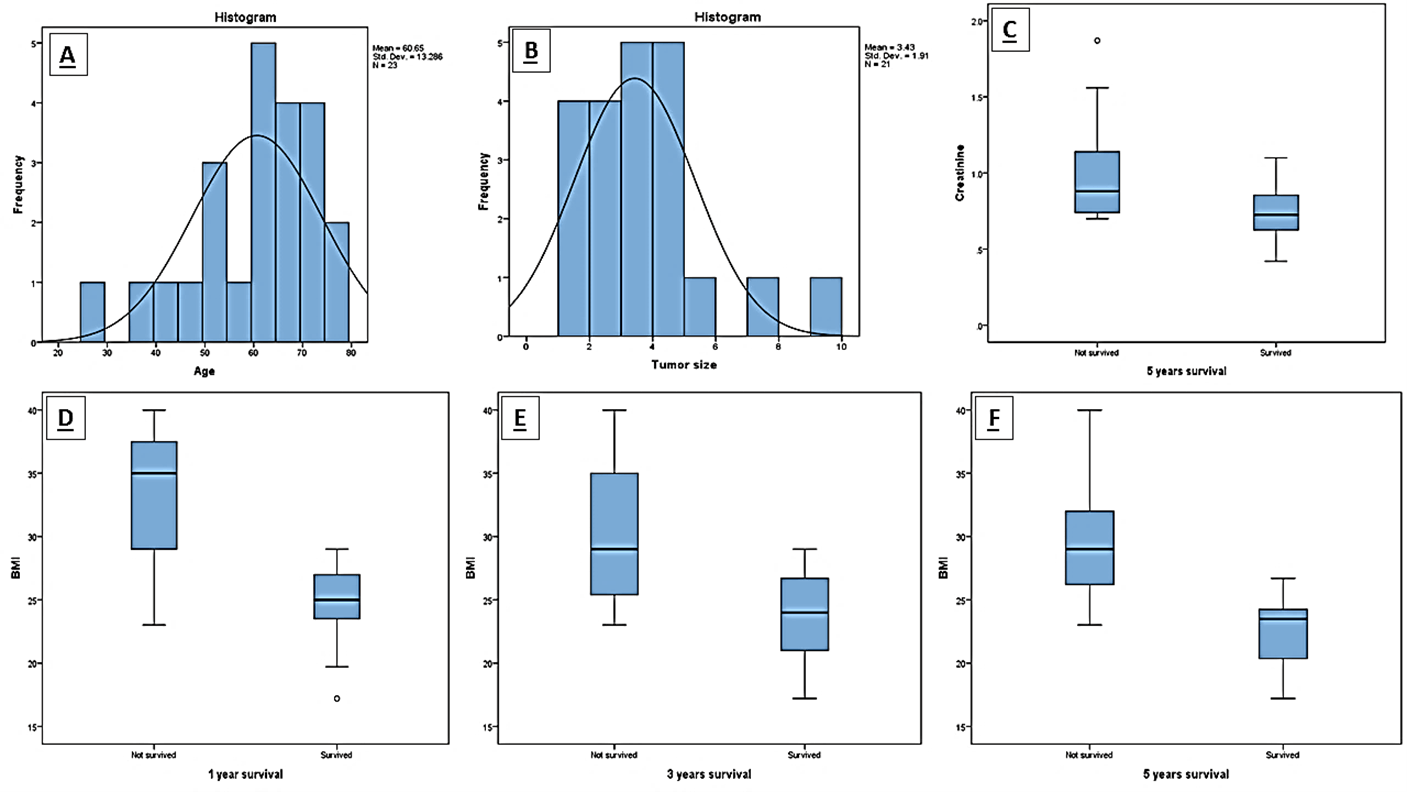
Figure 3: Histograms showing the repartition of A) Age and B) Survival in months, and C) the effect of Creatinine on 5 years’ survival, and D, E, F) BMI on 1, 3 and 5 years’ survival.
Results
I Patient Characteristics
23 patients were selected for this study. 2 patients were lost to follow-up and were excluded from subsequent statistical analysis. The mean age of the sample was 60.65 years of age. 10 patients underwent a right hepatectomy for BCT-IIIa tumors and 11 patients underwent a left hepatectomy for BCT-IIIb tumors.
II Surgical and Pathological Characteristics
R0 resection with lymph node dissection of the porta hepatis was achieved in 91.3% of cases (21 patients). The mean tumor size was 3.43cm, with the largest tumor measuring 9 cm along its longest axis and the smallest measuring 1cm. Final pathology revealed most to be moderately differentiated cholangiocarcinoma. Perineural invasion was observed in 78% of cases. Moreover, positive regional lymph nodes were observed in 40% of cases (Figure 2).
III Factors Predictive of Survival
Mean overall survival was 40.48 months with a mean disease-free survival of 38.85 months. Survival at 1, 3 and 5 years was 78.3%, 61.9% and 38.1% respectively. Preoperative body mass index (BMI) > 24 kg/m2 was significantly correlated with poorer overall survival at 1, 3 and 5 years (p-value = 0.02, p-value = 0.02 and p-value = 0.16 respectively). A creatinine > 0.74 at presentation was also significantly associated with decreased 5-year survival (p-value = 0.043) (Figure 3). A strong correlation was observed between age, sex and overall survival; however, results were deemed borderline significant for 1-year survival (p-value = 0.07 and p-value = 0.089 respectively). The use of a cavitron ultrasonic surgical aspirator (CUSA) and preoperative hemoglobin levels were observed to have an impact on overall survival (p-value = 0.073 and p-value = 0.088 respectively). Lastly, tumor size was observed to have an impact on disease free survival (p-value = 0.081).
IV Factors Predictive of Postoperative Bile Leak
PBL was observed in 43.5% of cases (10 patients). None of the patients developed infectious or septic complications secondary to the collection and none required surgical management. Spontaneous resolution between the 5th and 7th postoperative day was observed in 6 patients. 3 patients were discharged with a drain in place which was removed 3 weeks postoperatively to allow a mature fistulous tract to form. 1 patient required CT-guided drainage of the biloma and was discharged with a drain that was removed 3 weeks later. No statistically significant association was observed between the collected variables and the occurrence of a PBL. A borderline significant result was appreciated between BCT-IIIb tumors and the occurrence of a PBL, however (p-value = 0.074).
Discussion
Klatskin tumors remain to be a challenging entity to manage. With significant postoperative morbidity and mortality associated with curative resection, with rates of 14-66% and 0-19%, respectively, identifying predictive factors associated with complications and overall survival is crucial to better select surgical candidates and tailor postoperative care. In the literature, survival at 1, 3 and 5 years is 82%, 45% and 20%, respectively [5]. In our study, survival was 78.3%, 61.9% and 38.1%, respectively which is slightly higher for 3-year and 5-year survival. The statistical significance of this difference, however, was not assessed. In this study, we aimed to identify factors predictive of poor survival (overall survival and disease-free survival) as well as those predictive of PBL, a major cause of postoperative morbidity [9].
The prognostic role of various demographic, surgical, pathologic, and biochemical factors, both preoperatively and postoperatively, have been investigated in various studies, some demonstrating statistically significant associations while others do not. Classically, age, operating time and the type of surgery are predictive of poorer overall outcomes. In our study, only BMI and preoperative serum creatinine were significantly associated with poorer overall survivability, findings similar to those in the literature whereas age did demonstrate a strong correlation but was only borderline significant [9]. Furthermore, the association between left hepatectomies and PBL was also borderline significant. Similar associations were observed for sex, use of a CUSA, preoperative hemoglobin and tumor size where overall and disease-free survival is observed to be poorly impacted. However, the association was only borderline significant. These associations may be better appreciated in larger multicentric studies. Interestingly, in our study, none of the collected variables were demonstrated to be correlated with PBL. This contrasts the findings in the literature that reveals that more extensive surgery, prolonged operating time, advanced age, elevated biomarkers and BCT-IIIb tumors are more associated with increased occurrence of PBL. A borderline significant association was appreciated between BCT-IIIb tumors and PBL. However, this is best evaluation with a larger sample.
This study is not without limitations. The small sample size, chiefly due to the rarity of hCCA, limits both the statistical analysis and the generalizability of resulting associations. Moreover, the retrospective nature of the study design may create a certain selection bias. Prospective studies may not be feasible because of the low incidence of the disease. Therefore, the present and obtained associations are best investigated in large multicentric studies.
Conclusion
Perihilar cholangiocarcinoma is a challenging entity to manage and is associated with high postoperative morbidity and mortality. Various factors have been demonstrated to predict poorer outcomes, such as tumor histology, size, the extent of surgery, as well as demographic factors such as age and biochemical factors such as lymphocyte count and neutrophil-to-lymphocyte ratios. Elevated BMI > 24kg/m2 and preoperative serum creatinine > 0.74 are associated with poorer overall survival, whereas age, sex, preoperative hemoglobin levels, tumor size, use of a CUSA, and BCT-IIIb tumors were observed to influence overall survival but were only borderline significant. Furthermore, though many factors have been associated with PBL, no statistically significant association was appreciated in this study.
Acknowledgements
None.
Author Contributions
The authors made equal contributions to the following research article. AEA performed the literature review, drafted the manuscript and assisted in the data collection. Surgeon’s assistant. CAM and EG assisted in the data collection, drafting of the manuscript and the data analysis. TS and BA assisted in proofreading the manuscript and prepared the figures for the manuscript. ZAR formulated the research project, assisted in writing the final draft of the manuscript and provided approval for submission. Surgeon.
Conflicts of Interest
None.
Funding
None.
Consent
Verbal informed consent was obtained from the patient or his representative when applicable.
Ethical Approval
Institutional review board approval was obtained to conduct this study (Ref: IRB-REC/O/017-19/519).
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
hCCA: Hilar Cholangiocarcinoma
PBL: Postoperative Bile Leak
BCT-III: Bismuth-Corlette Type III
CT: Computed Tomography
MRCP: Magnetic Resonance Cholangiopancreatography
BMI: Body Mass Index
CUSA: Cavitron Ultrasonic Surgical Aspirator
Article Info
Article Type
Research ArticlePublication history
Received: Wed 21, Oct 2020Accepted: Thu 05, Nov 2020
Published: Mon 23, Nov 2020
Copyright
© 2023 Antoine R. El Asmar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.11.03
Author Info
Antoine R. El Asmar Christina Abou-Malhab Elie Ghabi Toufic Saber Bernard Akl Ziad El Rassi
Corresponding Author
Antoine R. El AsmarFellow in Digestive Surgery and Surgical Oncology, Institut Jules Bordet, Brussels, Belgium
Figures & Tables
FFP: Fresh Frozen Plasma; PRBC: Packed Red Blood Cells; CBC: Complete Blood Count; PT: Prothrombin Time; PTT: Partial thromboplastin time.
References
- Khan SA, Tavolari S, Brandi G (2019) Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 39: 19-31. [Crossref]
- Suarez Munoz MA, Fernandez Aguilar JL, Sanchez Perez B, Perez Daga JA, Garcia Albiach B et al. (2013) Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol 5: 132-138. [Crossref]
- Otani K, Chijiiwa K, Kai M, Ohuchida J, Nagano M et al. (2008) Outcome of surgical treatment of hilar cholangiocarcinoma. J Gastrointest Surg 12: 1033-1040. [Crossref]
- Neuhaus P, Thelen A (2008) Radical surgery for right-sided klatskin tumor. HPB (Oxford) 10: 171-173. [Crossref]
- Valero V, Cosgrove D, Herman JM, Pawlik TM (2012) Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol 6: 481-495. [Crossref]
- Bhuiya MR, Nimura Y, Kamiya J, Kondo S, Fukata S et al. (1992) Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 215: 344-349. [Crossref]
- Ouchi K, Suzuki M, Hashimoto L, Sato T (1989) Histologic findings and prognostic factors in carcinoma of the upper bile duct. Am J Surg 157: 552-556. [Crossref]
- Sakamoto E, Nimura Y, Hayakawa N, Kamiya J, Kondo S et al. (1998). The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 227: 405-411. [Crossref]
- Dumitrascu T, Brasoveanu V, Stroescu C, Ionescu M, Popescu I (2016) Major hepatectomies for perihilar cholangiocarcinoma: Predictors for clinically relevant postoperative complications using the International Study Group of Liver Surgery definitions. Asian J Surg 39: 81-89. [Crossref]
- Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K et al. (2001) Bile leakage after hepatic resection. Ann Surg 233: 45-50. [Crossref]
- Nagano Y, Togo S, Tanaka K, Masui H, Endo I et al. (2003) Risk factors and management of bile leakage after hepatic resection. World J Surg 27: 695-698. [Crossref]
- Lo C M, Fan S T, Liu C L, Lai E C, Wong J (1998) Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg 133: 156-161. [Crossref]
- Baton O, Azoulay D, Adam D V, Castaing D (2007) Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg 204: 250-260. [Crossref]
- Berardi R, Mocchegiani F, Pierantoni C, Federici A, Nicolini D et al. (2013) Resected biliary tract cancers: a novel clinical-pathological score correlates with global outcome. Dig Liver Dis 45: 70-74. [Crossref]
- Kaiser GM, Paul A, Sgourakis G, Molmenti EP, Dechêne A et al. (2013) Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg 79: 90-95. [Crossref]
- Korkmaz C, Choucair S, Irani J, Saikaly E, El Rassi Z (2016) Operated Klatskin tumors: A series of 22 patients in a single center in Lebanon. Int J Hepatobiliary Pancreatic Dis 6: 34-42.
- Saito H, Noji T, Okamura K, Tsuchikawa T, Shichinohe T et al. (2016) A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery 159: 842-851. [Crossref]
- Olthof P B, Wiggers J K, Groot Koerkamp B, Coelen R J, Allen P J et al. (2017) Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J Am Coll Surg 225: 387-394. [Crossref]
- Brown KM, Geller DA (2014) Proximal biliary tumors. Surg Clin North Am 94: 311-323. [Crossref]
- Lee HY, Kim SH, Lee JM, Kim SW, Jang JY et al. (2006) Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 239: 113-121. [Crossref]
