Proceeding of Glioblastoma Multiforme (GBM): An Advanced Overview
Proceeding of Glioblastoma Multiforme (GBM): An Advanced Overview
A B S T R A C T
Glioblastoma multiforme (GBM) is a central nervous system tumor of grade IV histological malignancy according to the WHO classification. GBM is an extraordinary tumor and the worldwide incidence is less than 10 per 100,000 individuals. The prognosis of this tumor is meagre and survival rate is 15 months subsequent to diagnosis. Such an atrocious image of this tumor is responsible for a major issue of public health. This tumor develops from normal glial cells through multistep oncogenesis. Genetic alterations and deregulations of molecular pathways are responsible for development of both primary and secondary GBM. Diagnosis of GBM is performed by imaging techniques. These non-invasive imaging techniques are magnetic resonance imaging (MRI), computed tomography (CT) and Positron emission tomography (PET). Definitive diagnosis of GBM is based on histopathological examination of the intra operatively removed tumor or its parts, using traditional histological, cytologic and histochemical methods. NMR (Nuclear Magnetic Resonance) spectroscopy based possible diagnostic significance of GBM is also reported. Treatment of glioblastoma multiforme includes tumor resection, as well as radiotherapy and chemotherapy. Nanomedicine and bacterial protein based therapeutic approaches are in the developmental stage. There is always need of the development of new therapeutic approaches for GBM to improve the survival and quality of life.
Keywords
Glioblastoma multiforme, primary brain tumors, high-grade tumor, grade IV tumor, central nervous system tumor, brain cancer
Glioblastoma Multiforme (GBM): Brief Introduction
Primary brain tumors represent a heterogeneous group of disorders that diverge from benign, slow-growing lesions to aggressive malignancies. In general, the primary brain tumors are arising from glial cells, are considered as gliomas. The most malignant and lethal form of glioma is glioblastoma multiforme (GBM). GBM develops from astrocytes and is the generally recurrent malignant brain tumor [1-2]. Initial therapeutic approach for patient with GBM is surgery. Surgical procedure is not curative and in general a combination of radiotherapy and chemotherapy is also provided [2-3]. In spite of the diversity of advanced therapies adjacent to GBM, but till date this is a fatal disease with enormously deprived prognosis. In general, the median survival of patient with GBM is around 15 months and rarely goes beyond 2 years after the day of diagnosis [1-5].
Epidemiology
GBM is a extraordinary tumor and the worldwide incidence is less than 10 per 100,000 individuals. The prognosis of this tumor is meagre and survival rate is 15 months subsequent to diagnosis. Such an atrocious image of this tumor is responsible for a major issue of public health [6-7]. GBM covers 50% of the entire gliomas in all age groups [8]. This tumor can observe at all age, however the peak frequency is between 55 to 60 years [9]. Incidence ratio of GBM is higher in male as compares to female [7, 9]. Several authors reported the higher incidence of gliomas in western and developed countries as compared to poor and less developed countries [7]. The reason behind this could be due to a reduced reporting of gliomas cases, deprived health care facilities and inconsistency in diagnostic practices [10-11]. A small number of reports have shown that African origin peoples are not as much of prone of GBM as compared to other ethnic groups together with Asians, Latinos and Europeans [6].
GBM Grading
Grading of gliomas has been performed by World Health Organization (WHO). The WHO graded the gliomas from I - IV and the basis of grading is histological characteristics, prognosis and median age of survival. According to WHO grading, GBM is a grade IV glioma. The recent international standard for the nomenclature and diagnosis of gliomas is WHO based categorization. Grading of gliomas into grade I to IV is also provided the level of malignancy that is determined by the histopathological criteria. Grade I gliomas are less malignant and have low proliferative potential, whereas grade II to IV gliomas are extremely malignant and invasive. GBM (grade IV) is the largely aggressive, invasive and undifferentiated type of tumor [12-14].
Clinical Presentation of GBM [Symptoms and Signs]
Clinical presentation of patients with GBM may appear with different signs and symptoms. These signs and symptoms are derived by three mechanisms [1]. In first mechanism, the straightforward influence on brain tissue. In such mechanism, destruction of the brain tissue is carried out in the form of necrosis, which is responsible for appearing the symptoms such as focal neural deficit (40-60%) and cognitive impairments. A region of the brain, which is affected by the tumor, is also responsible for appearance of signs and symptoms. Patients with GBM, who demonstrate hearing and visual problems, specify that a tumor is located in the temporal lobe area. A total of 20-40% GBM patients showed the personality change with impairing cognitive functions (as a consequence of tumor located in their frontal lobe). Larger size tumor is responsible for considerable to imbalance in gait and incontinence [1, 15-16].
In another mechanism is appeared in the form of secondary effects on the brain tissue. Enhancement of intracranial pressure is a direct outcome of gradual enlarges in tumor size and also increased oedema surrounding the tumor. This event is appeared in the form of symptom of headaches, which is a characteristic feature in 30-50% of GBM patients [1, 15]. Headaches are frequently and localized unilaterally with progressive severity and having no specific pain pattern. A rare symptom of headaches may also be associated with vomiting and papilledema [16].
In another mechanistic effect on brain is responsible for symptom of seizures usually with a focal onset. Location of tumor in 20-40% of the GBM cases may also present simple partial, complex partial or generalised seizures [1, 15-16].
Pathogenesis of GBM
Sophisticated technology of genomics has explained the molecular alterations-based pathogenesis of GBM. [1, 17-18]. Pathological characteristics of GBM can be subdivided into primary and secondary GBMs. Primary GBMs has observed de novo without clinical and histological evidence of precursor lesion. The secondary GBMs develops gradually from pre-existing lower-grade astrocytoma [19-20]. Characteristic alterations of primary GBM include epidermal growth factor receptor (EGFR) gene mutation and amplification, over expression of mouse double minute 2 (MDM2), deletion of p16 and loss of heterozygosity (LOH) of chromosome 10q holding phosphatase and tensin homolog (PTEN) and TERT promoter mutation. The characteristic features of secondary GBMs include over expression of platelet-derived growth factor A, and platelet-derived growth factor receptor alpha (PDGFA/PDGFRa), retinoblastoma (RB), LOH of 19q and mutations of IDH1/2, TP53 and ATRX [20-24].
The depth and evidence-based investigation of the several genetic abrasions has proved that these genetic lesions are grouped into three main signalling pathways. These are receptor tyrosine kinase/RAS/PI3K (distorted in almost 88% of GBMs cases), P53 pathway (distorted in 87% of GBMs) and RB signalling pathway (distorted in approximately 78% of GBMs) [25]. Transcriptional subclass of GBM has also started to come forward from global gene expression studies. The hallmarks of proneural transcriptional subclass are CDK4, CDK6, PDGFRA, MET and the most frequent IDH1 mutations. Classical subtype is categorized by the loss of PTEN and CDKN2A and EGFR amplification [24, 26].
Diagnostic Approaches of Glioblastoma
Diagnosis of GBM is performed by imaging techniques. These non-invasive imaging techniques are magnetic resonance imaging (MRI), computed tomography (CT) and Positron emission tomography (PET) [1, 27-28]. Magnetic resonance imaging (MRI), the current imaging gold standard, has offered limited insight to date with regard to grade of malignancy, tumor delineation, differentiation between necrotic tissues and recurrent tumor, as well as the management of therapeutic interventions such as surgery or radiotherapy [27-28]. MRI has been applied for more than 20 years to accomplish a clinical diagnosis of GBM. This is also used to monitor the therapy of GBM [29].
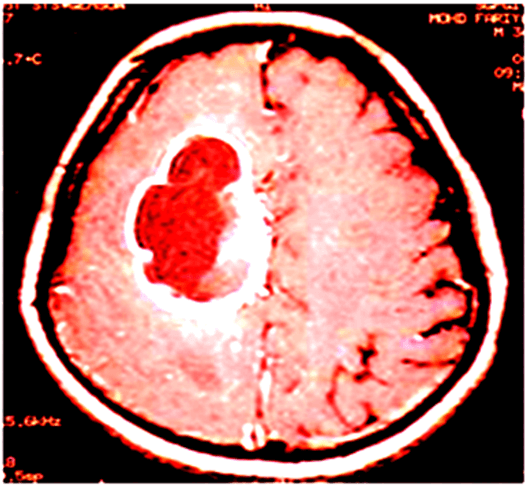
Figure 1: T1 weighted with contrast enhancement image showed a ring-enhancing necrotic tumor in the right frontal lobe.
CT scans are performed on such patients, who cannot undergo MRI due to specific reasons, for example, patients with pacemakers [30]. CT scan-based imaging showed the lesions in the hypointense areas as compared to adjacent brain tissue and generally reveals a midline shift as a consequence of moderate to severe oedema. MRI scans showed the better visualization of the complexity and the heterogeneity of the tumor lesion as compared to CT scan because of the superior soft tissue contrast of MRI. T1- weighted of MRI scans noticeably visualized the hypointense lesions (Figure 1), whereas hyper intense lesions are visualized on proton density weighted and T2-weighted images [1]. The observation on a MRI scan improved with gadolinium of patients with GBM shows a central area of necrosis, surrounded by white matter [30].
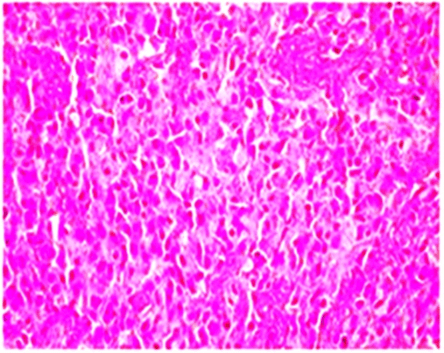
Positron emission tomography (PET) is a nuclear medicine based imaging approach with an enhanced significance especially due to the improved availability of radioactively labelled amino acids, permitting extensive applications in diagnosis, planning of surgery, radiotherapy, post-treatment monitoring and prognostication of glioblastomas [27-28, 31]. Definitive diagnosis of GBM is based on histopathological examination of the intra operatively removed tumor or its parts, using traditional histological, cytologic and histochemical methods. A predominant population of highly proliferative small cells can be seen in the variant form called small cell glioblastoma. The predominance of such cells often raises a differential diagnosis of small blue cell tumors including malignant lymphoma. Although cellular pleomorphism is an inherent histological feature of glioblastoma, an extremely pleomorphic variant with florid multinucleated giant cells constitutes giant cell glioblastoma (Figure 2) [32-33].
Figure 2: Marked cellular pleomorphism, frequent mitotic figures, tumor giant cells and endothelial proliferation with necrosis.
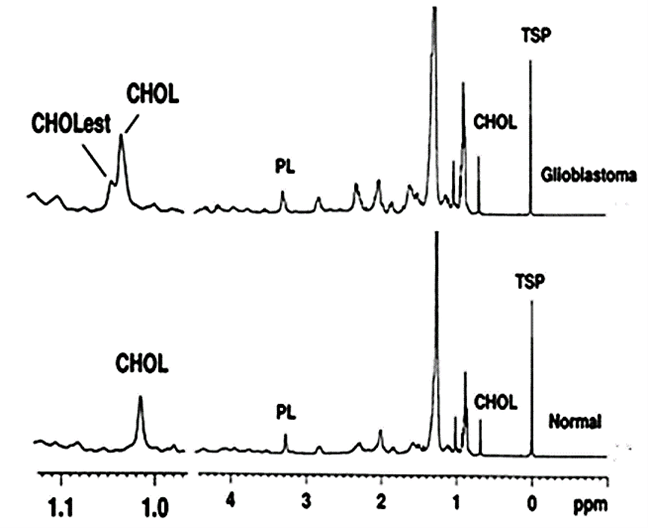
Figure 3: 1H NMR spectra of the lipid extract of the surgically discarded tissue of GBM and normal brain showed the enhanced level of free cholesterol (CHOL) and cholesterol esters (CHOLest) in GBM as compared to normal [PL: phospholipid; TSP: 3-(trimethylsilyl) propionic-2, 2, 3, 3-d4 acid, sodium salt].
NMR (Nuclear Magnetic Resonance) spectroscopy based possible diagnostic significance of GBM is also reported. This NMR-based study of the lipid profile of tissue (Figure 3), serum, and CSF of patients with GBM serve as a possible diagnostic tool. Outcomes may provide supportive evidence to the histopathological examination or even become established in future as the supportive technique for preoperative assessment of tumor subtypes and tumor grades, which often helps in deciding the type of surgery [34].
Therapeutic Approaches of Glioblastoma
Recent trend of therapeutic approaches involves total surgical resection followed by combination of radiotherapy with temozolomide and the continued adjuvant temozolomide therapy. The radiation therapy for GBM is carried out in the form of focal, fractionated external beam therapy. The recent drugs used for GBM patients are temozolomide (alkylating agents) and erlotinib (inhibitors of epidermal growth factor receptor). These two drugs are used in a combination [35]. By applying such combination therapy, the survival of GBM patients has not been enhanced, representing a need for better therapeutic intervention. The major problem to the brain of several drugs proposed for the treatment of brain pathologies have triggered a flurry of performances to design drugs (lipophilic compounds with polar groups) that can cross the blood-brain barrier (BBB) to penetrate the brain parenchyma by disrupting the tight junctions of the endothelial cells of the brain capillaries. Elimination of drug entry problem are being made to use devices that can straight forward transport a drug to the tumor. Carmustine impregnate wafer can be positioned in the cavity after tumor resection or other anticancer agents can be applied in drug-eluting catheters positioned in the cavity after surgery. The unsuccessful of several agents (including monoclonal antibodies such as Avastin that target the vascular endothelial growth factor) and other antiangiogenic agents that strike a particular target, eliciting rapid drug resistance as well as arising the problems of considerable toxicity [36]. To eliminate such difficulties or problems, there is a need of an innovative therapeutic approach and a new conceptual framework for the treatment of GBM [37-38].
Several attempts of researchers in worldwide are carried out for therapeutic approach of GBM, but the therapeutic approach is still the most challenging task in clinical oncology. Over the last decade, several therapeutic approaches were investigated with extremely inadequate success. The major challenges in therapeutic approach of GBM are connected with the location of the disease, complexity and heterogeneous biology [1].
Nanomedicine based therapeutic approach are developed for GBM. Under this approach, systematically experiments were performed to observe the effects of different anthocyanin derivatives on GBM progression. Outcomes of these experiments were hopeful and again promote the development of a computational model for the delivery of direct and encapsulated forms of delphinidin (anthocyanin derivatives) to GBM through the BBB. Specifically, two models developed: (1) to predict delivery of delphinidin by itself and (2) to predict delivery of nano particle encapsulated forms of delphinidin to brain tissue. Such type of attempt again opens a new hope in the GBM treatment [39].
Another new therapeutic approach for GBM is application of bacterial proteins and peptides in potential GBM therapy. An azurin-like protein is also known as Laz derived from Neisseria meningitides reveals competent entrance and high cytotoxicity towards glioblastoma cells. Laz diverges from azurin in having an extra 39-amino-acid peptide called an H.8 epitope, which permits entrance and high cytotoxicity towards glioblastoma cells. While p28 has been shown to have very small toxicity and high anti-tumor activity in advanced-stage cancer patients, it will be worthwhile to explore the use of H.8-p28, H.8-azurin, and Laz in toxicity studies and glioblastoma therapy in preclinical and human clinical trials [40].
Conclusion
Highly lethal form of brain tumor is glioblastoma multiforme (GBM) and this is characterized by rapid growth and invasion facilitated by cell migration and degradation of the extracellular matrix. Notwithstanding technological advances in surgery and radio-chemotherapy, GBM remains largely resistant to treatment. There is always need of the development of new therapeutic approaches for GBM to improve the survival and quality of life.
Acknowledgements
Authors wish to thank Council of Scientific & Industrial Research [No.13 (8660-A)/2013-Pool] and University Grant Commission [No.F.4-2/2006 (BSR)/ 13-194/2008(BSR)], Government of India, for their generous financial support. Professor Rajkumar (Department of neurosurgery, SGPGIMS, Lucknow) is acknowledged for providing the operated GBM tissue.
Abbreviations
GBM: Glioblastoma Multiforme
MRI: Magnetic Resonance Imaging
CT Scan: Computer Tomography Scan
PET: Positron Emission Tomography
WHO: World Health Organization
BBB: Blood Brain Barrier
Article Info
Article Type
Research ArticlePublication history
Received: Sat 15, Aug 2020Accepted: Mon 31, Aug 2020
Published: Mon 28, Sep 2020
Copyright
© 2023 Niraj Kumar Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COCB.2020.02.02
Author Info
Niraj Kumar Srivastava Somnath Mukherjee
Corresponding Author
Niraj Kumar SrivastavaDepartment of Neurology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Figures & Tables
References
- Farina Hanif, Kanza Muzaffar, Kahkashan Perveen, Saima M Malhi, Shabana U Simjee (2017) Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J Cancer Prev 18: 3-9. [Crossref]
- M Touat, A Idbaih, M Sanson, K L Ligon (2017) Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol 28: 1457-1472. [Crossref]
- Lingqi Zhou, Hai Tang, Fang Wang, Lizhi Chen, Shanshan Ou et al. (2018) Bioinformatics analyses of significant genes, related pathways and candidate prognostic biomarkers in glioblastoma. Mol Med Rep18: 4185-4196. [Crossref]
- Alexander Baraniskin, Jan Kuhnhenn, Uwe Schlegel, Abdelouahid Maghnouj, Hannah Zöllner et al. (2012) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14: 29-33. [Crossref]
- Adomas Bunevicius, Nathan Judson McDannold, Alexandra J Golby (2020) Focused Ultrasound Strategies for Brain Tumor Therapy. Oper Neurosurg (Hagerstown) 19: 9-18. [Crossref]
- Gabriel Iacob, Eduard B Dinca (2009) Current data and strategy in glioblastoma multiforme. J Med Life 2: 386-393. [Crossref]
- Jigisha P Thakkar, Therese A Dolecek, Craig Horbinski, Quinn T Ostrom, Donita D Lightner et al. (2014). Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 23: 1985-1996. [Crossref]
- K Rock, O McArdle, P Forde, M Dunne, D Fitzpatrick et al. (2012) A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival? Br J Radiol 85: e729-e733. [Crossref]
- Hiroko Ohgaki, Paul Kleihues (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109: 93-108. [Crossref]
- James L Fisher, Judith A Schwartzbaum, Margaret Wrensch, Joseph L Wiemels (2007) Epidemiology of brain tumors. Neurol Clin 25: 867-890. [Crossref]
- Hiroko Ohgaki (2009) Epidemiology of brain tumors. Methods Mol Biol 472: 323-342. [Crossref]
- Giulia Regazzo, Irene Terrenato, Manuela Spagnuolo, Mariantonia Carosi, Gaetana Cognetti et al. (2016) A restricted signature of serum miRNAs distinguishes glioblastoma from lower grade gliomas. J Exp Clin Cancer Res 35: 124. [Crossref]
- Erik P Sulman, Marisol Guerrero, Ken Aldape (2009) Beyond grade: molecular pathology of malignant gliomas. Semin Radiat Oncol 19: 142-149. [Crossref]
- Song Xue, Man Hu, Veena Iyer, Jinming Yu (2017) Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol 10: 81. [Crossref]
- Clarke CRA (2005) Neurological diseases in Kumar & Clark. Clinical Medicine. Kumar P and Clark M. 6th ed. Elsevier Saunders, Edinburgh 1244-1245.
- Antonio Omuro, Lisa M DeAngelis (2013) Glioblastoma and other malignant gliomas: a clinical review. J Am Med Assoc 310: 1842-1850. [Crossref]
- David N Louis, Hiroko Ohgaki, Otmar D Wiestler, Webster K Cavenee, Peter C Burger et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97-109. [Crossref]
- Timothy F Cloughesy, Webster K Cavenee, Paul S Mischel (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9: 1-25. [Crossref]
- Smith C, Ironside JW (2007) Diagnosis and pathogenesis of gliomas. Curr Diagn Pathol 13: 180-192.
- Sameer Agnihotri, Kelly E Burrell, Amparo Wolf, Sharzhad Jalali, Cynthia Hawkins et al. (2013) Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp (Warsz) 61: 25-41. [Crossref]
- Hiroko Ohgaki, Paul Kleihues (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170: 1445-1453. [Crossref]
- Hiroko Ohgaki, Paul Kleihues (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19: 764-772. [Crossref]
- Xiao Yang Liu, Noha Gerges, Andrey Korshunov, Nesrin Sabha, Dong Anh Khuong Quang et al. (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124: 615-625. [Crossref]
- Timothy F Cloughesy, Webster K Cavenee, Paul S Mischel (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9: 1-25. [Crossref]
- Kenneth Aldape, Gelareh Zadeh, Sheila Mansouri, Guido Reifenberger, Andreas von Deimling (2015) Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129: 829-848. [Crossref]
- Roel G W Verhaak, Katherine A Hoadley, Elizabeth Purdom, Victoria Wang, Yuan Qi et al. (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17: 98-110. [Crossref]
- Wei Chen, Daniel H S Silverman (2008) Advances in evaluation of primary brain tumors. Semin Nucl Med 38: 240-250. [Crossref]
- W D Heiss (2014) PET in gliomas. Overview of current studies. Nuklearmedizin 53: 163-171. [Crossref]
- M A Hammoud, R Sawaya, W Shi, P F Thall, N E Leeds (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neuroncol 27: 65-73. [Crossref]
- Antonio Omuro, Lisa M DeAngelis (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310: 1842-1850. [Crossref]
- Frederic Dhermain (2014) Radiotherapy of high-grade gliomas: Current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer 33: 16-24. [Crossref]
- C Ryan Miller, Arie Perry (2007) Glioblastoma. Arch Pathol Lab Med 131: 397-406. [Crossref]
- Kleihues P, Burger PC, Scheithauer BW, Zu¨ lch KJ (1993) Histological Typing of Tumours of the Central Nervous System. 2nd ed. Berlin, Germany: Springer-Verlag; xii, 112.
- Niraj Kumar Srivastava, Sunil Pradhan, G A Nagana Gowda, Raj Kumar (2010) In vitro, high-resolution 1H and 31P NMR based analysis of the lipid components in the tissue, serum, and CSF of the patients with primary brain tumors: one possible diagnostic view. NMR Biomed 23: 113-122. [Crossref]
- Michael D Prados, Susan M Chang, Nicholas Butowski, Rebecca DeBoer, Rupa Parvataneni et al. (2009) Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 27: 579-584. [Crossref]
- Andrew S Chi, Andrew D Norden, Patrick Y Wen (2009) Antiangiogenic strategies for treatment of malignant gliomas. Neurotherapeutics 6: 513-526. [Crossref]
- Tiffany T Huang, Shawn M Sarkaria, Timothy F Cloughesy, Paul S Mischel (2009) Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics 6: 500-512. [Crossref]
- Ren Yuan Bai, Verena Staedtke, Gregory J Riggins (2011) Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med 17: 301-312. [Crossref]
- Elif Ozdemir Kaynak, Amina A Qutub, Ozlem Yesil Celiktas (2018) Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front Physiol 9: 170. [Crossref]
- A M Fialho, A M Chakrabarty (2010) Promiscuous anticancer drugs from pathogenic bacteria: rational versus intelligent drug design. Emerging Cancer Therapy: Microbial Approaches and Biotechnological Tools, Eds., 181-198.
