Journals
Primary Typical Carcinoid of The Pleura, A Rare Incidental Finding
A B S T R A C T
Roughly 30% of carcinoids are found in the thoracic cavity, with the overwhelming majority of these being found in the lung parenchyma. We present a case of a rarely found primary pleural carcinoid incidentally diagnosed in a 73 year old male who presented with recalcitrant spontaneous pneumothorax requiring surgical intervention. The patient was taken to the operating room for a right video assisted thoracic surgery, partial pleurectomy, and talc pleurodesis. At the time of the operation, there were no abnormalities noted in the pleura or chest wall, and the patient did well post operatively. Pathologic examination of the pleura revealed a 3mm well differentiated neuroendocrine carcinoid tumor. Laboratory and imaging studies did not indicate another site of tumor. The importance of a multidisciplinary approach to the diagnosis, treatment, and surveillance is emphasized.
Keywords
video assisted thoracoscopic turgery, neuroendocrine carcinoid tumor, multidisciplinary tumor board
Introduction
Though 30% of all carcinoid tumors are of pulmonary origin, carcinoid tumors are a rare occurrence of the thoracic cavity, constituting roughly 0.5 to 2.5% of all pulmonary neoplasms. They are often described as a malignant neoplasm of neuroendocrine origin with the ability to produce endogenous hormones. These tumors are often categorized as either typical or atypical, a diagnosis that is fueled by several histologic features, which include mitoses per high powered field, nuclear pleomorphism, and necrosis [1]. An overwhelming majority of thoracic carcinoid tumors arise from the pulmonary parenchyma or airway. However, it is rare for a carcinoid tumor to originate in the parietal pleura. This case discusses an incidental finding of a primary pleural carcinoid in a patient presenting with spontaneous pneumothorax requiring operative management.
Case Report
A 73-year-old male with a history of chronic obstructive pulmonary disease was transferred from an outside facility with a spontaneous right pneumothorax. Approximately one week prior, he was being treated for an upper respiratory tract infection and had several episodes of severe coughing. He initially presented to a local emergency department with chest pain and shortness of breath. A chest x-ray revealed a right pneumothorax and he was treated with an 8 French pleural catheter. After failing several water-seal trials, the patient was transferred to our facility under the thoracic surgery service. At that time, he had no hemoptysis, fevers, chills, or shortness of breath.
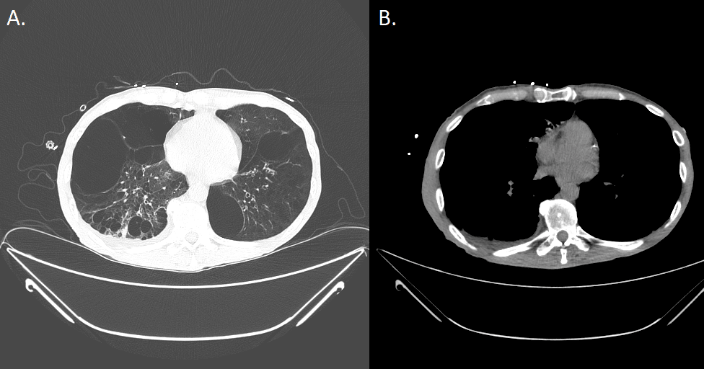
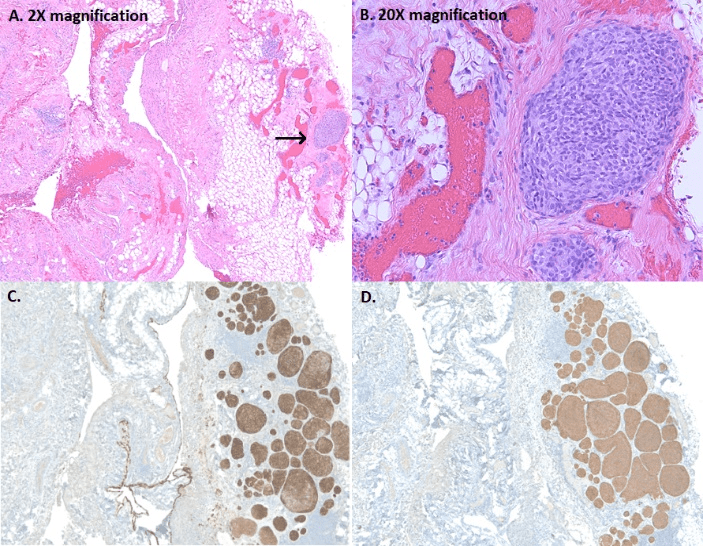
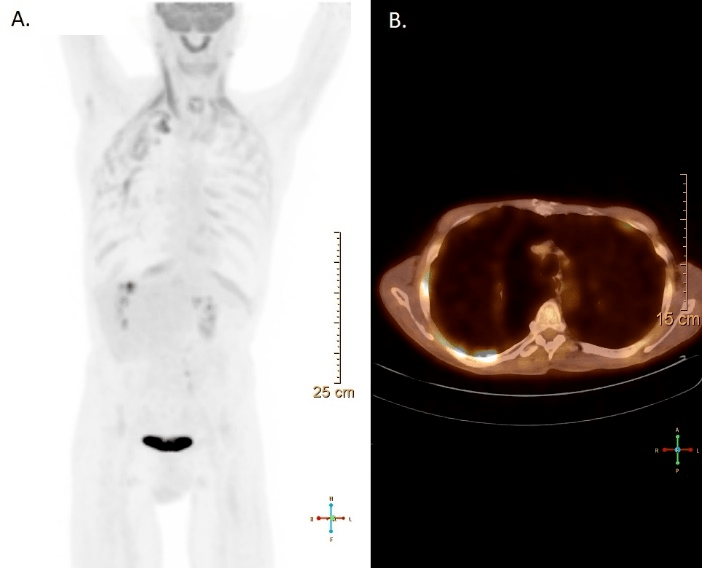
A CT scan revealed multiple bilateral emphysematous blebs, but no pulmonary or chest wall lesions (Figure 1). Over the course of the next several days, the pneumothorax was persistent despite the upsizing of the pleural drain to a 28 French chest tube. Despite increasing the suction to -40 mmHg and placing a second chest tube, the patient developed subcutaneous emphysema extending to his face. The patient was taken to the operating room for a right video assisted thoracic surgery, partial pleurectomy, and talc pleurodesis. Intraoperatively, there were no obvious blebs or areas of air leak despite multiple attempts to identify them under saline. No abnormalities were noted in the pleura or chest wall at the time of pleurectomy. The patient tolerated the procedure well and two chest tubes were placed intraoperatively. Both chest tubes were placed to water-seal on POD 3, and were removed 2 days later. The patient was discharged home on postoperative day 7. The specimen was described as an irregularly shaped yellow transparent membrane measuring 14.5 cm X 0.5 cm X 0.1 cm. There were no lesions that were grossly identified. However, microscopic analysis revealed a 3 mm well differentiated neuroendocrine carcinoid tumor. Nests of neuroendocrine cells were present in the chest wall fat and stained positive for CD-56, CK7, and synaptophysin. CEA was focally positive as well. Several other molecular tests including ERG, CK20, WT-1, cam 5.2, TTF-1, CDX-2, and calretinin were negative (Figure 2). At the time of follow up, the patient was recovering well with a PET-CT identifying post-surgical changes without areas suspicious for additional tumor (Figure 3).
Figure 1A-B: Pre-operative CT scan of the chest depicting multiple bilateral emphysematous blebs bilaterally with no evidence of any pleural disease
Figure 2: A-B: Focal nodular proliferation of neuroendocrine cells in a background of chronic pleuritis; C-D: Diffuse strong immunoreactivity for Cytokeratin 7 (epithelial marker) and synatophysin (neuroendocrine marker), within the pleural carcinoid.
Discussion
Carcinoid tumors are neuroendocrine tumors that typically arise from Kultchitsky cells. They comprise of 2.5% of all primary lung tumors [1]. These malignancies are categorized as typical or atypical by various histopathologic characteristics, with 90% of lesions being typical. Typical carcinoids, unlike their counterpart, rarely metastasize and therefore have a better prognosis. The most common primary location of carcinoid tumors is the small intestine (30.4%), followed by lung (29.8%) and colon (9.2%) [1]. A majority of reported pleural cases are of metastatic disease [2-4]. Therefore, primary pleural carcinoid disease is a rarity. Computed tomography can be used as the imaging modality to identify thoracic or gastrointestinal primary carcinoid tumors. In a retrospective review of 50 patients with carcinoid syndrome, Moss et al. identified pleural thickening in nine patients. Four of these patients had a primary tumor with the remaining five having an unknown primary site. Presentation of these patients with primary pleural carcinoid is usually due to mass effect or as an incidental finding on imaging [3].
Figure 3: Follow-up PET-CT identifying post-surgical changes without areas suspicious for additional tumor
Given the rarity of primary pleural carcinoid, this finding warranted further investigation to ensure that this was not metastatic disease. Metastatic carcinoid tumors often respond dramatically to chemotherapeutic treatments. A multidisciplinary approach to these rare cases with pathology and radiology cannot be stressed enough. The first step is to rule out other more common pleural malignancies though a panel of histologic stains. Further imaging and laboratory testing is recommended to illicit information regarding the presence of a potential primary lesion. Lastly, future surveillance with additional imaging at the time of office visits is warranted for the evaluation of any new symptoms.
Disclosures
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article Info
Article Type
Case ReportPublication history
Received: Thu 30, May 2019Accepted: Tue 18, Jun 2019
Published: Thu 29, Aug 2019
Copyright
© 2023 Rajeev Dhupar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJSCR.2019.02.02
Author Info
Anupama Sharma Diane Strollo Ernest G. Chan James D. Luketich Lawrence Crist Rajeev Dhupar
Corresponding Author
Rajeev DhuparDepartment of Cardiothoracic Surgery, University of Pittsburgh School of Medicine
Figures & Tables
References
- Bertino EM, Confer PD, Colonna JE, Ross P, Otterson GA (2009) Pulmonary neuroendocrine/carcinoid tumors. Cancer 115: 4434-4441. [Crossref]
- Townshend AP, Lakshminarayanan B, Ucar AE, Chaudry ZR, Duffy JP (2007) Rare pleural recurrence of typical pulmonary carcinoid tumor 30 years after lobectomy. Ann Thorac Surg 83: 1523-1524. [Crossref]
- Moss SF, Lehner PJ, Gilbey SG, Kennedy A, Hughes JMB et al. (1993) Pleural involvement in the carcinoid syndrome. Q J Med 86: 49-53. [Crossref]
- Klee H, Vestring T, Bittmann I (2008) [Pleural metastases of a typical bronchial carcinoid 7 years after lobectomy]. Pneumologie 62: 607-610. [Crossref]
