Primary Localised Amyloidosis of the Urethra: A Case Report
A B S T R A C T
Primary localised amyloidosis of the urethra is a rare entity with only 50 cases reported in the literature. The deposition of fibrillary proteins can lead to a range of symptoms. Importantly, the clinical and cystoscopic features may mimic urethral malignancy (haematuria, voiding difficulties, and a palpable urethral mass), and so thorough investigation is required in order to exclude malignancy, and to identify features of generalised amyloidosis which has a poor prognosis. Once diagnosed the prognosis of localised amyloidosis is excellent, although disease recurrence is possible. We describe a case of primary localised urethral amyloidosis presenting with visible haematuria and a urethral stricture and reviewed the literature regarding management of this rare condition.
Keywords
Amyloidosis, urethral stricture, haematuria
Introduction
Amyloidosis is a rare group of diseases characterized by deposition of proteinaceous substances in the extra-cellular tissue. The composition of these deposits may vary. Clinical manifestations vary depending on the extent of involvement of the tissues as localized or systemic. Amyloidosis can be classified based on chemical composition of the deposits (biochemical classification) or clinical extent (clinical classification). The generalised and localised patterns of the condition could be further sub-classified into primary amyloidosis which is associated with immunocyte dyscrasias (AL), or secondary which usually results from chronic inflammatory diseases or tissue destructive processes (AA) [1]. Involvement of the lower urinary tract with amyloidosis is rare. To date about 50 cases of primary urethral amyloidosis have been reported in literature [2]. All were males except one female. Patients with primary amyloidosis of the urethra may present with a variety of symptoms, but most commonly the disease mirrors either non-specific urethritis or a primary urethral tumor. We report a case of primary urethral amyloidosis associated with urethral stricture in a young male who presented with visible haematuria. A review of the available literature is also presented.
Case Report
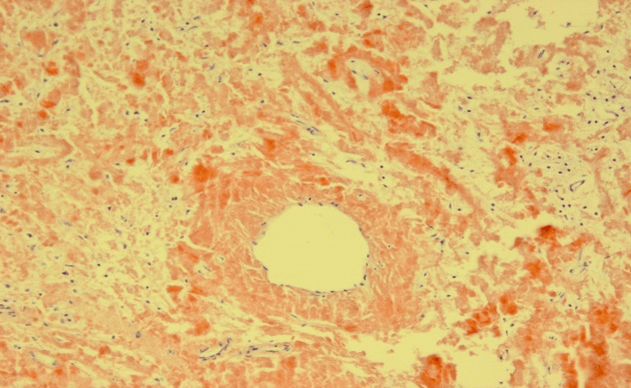
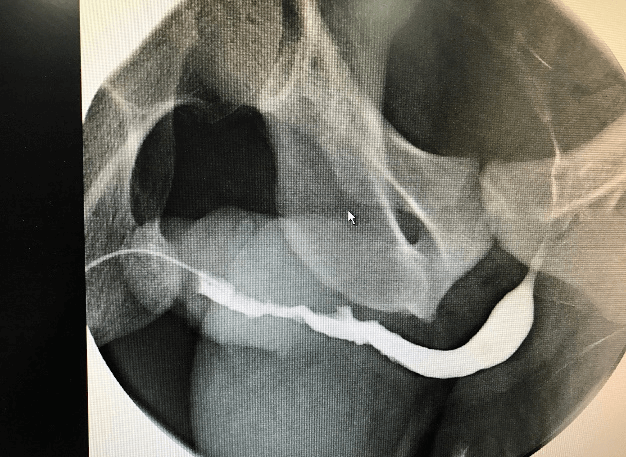
A 34-year-old male presented to the emergency room at his local hospital with visible haematuria. Flexible urethroscopy demonstrated a white patch in the penile urethra with a proximal urethral stricture. He subsequently underwent urethral dilatation and biopsy of the lesion under general anaesthesia. The histology was reported as squamous metaplasia at the referring hospital. The patient was then transferred for further management to our specialist urethral disease service. As per protocol the pathology slides were reviewed, and further special stains (Congo Red) were carried out. Based on these, the diagnosis was revised as primary amyloidosis of the urethra (Figure 1). An ascending urethrogram showed generalised penile urethral stricture (Figure 2). A repeat diagnostic urethroscopy was carried out using an 8Fr ureteroscope showing an irregular and narrowed penile urethral lumen. The remainder of the urethra was wide open. After urethral dilatation a 19Fr cystoscope was used to examine the proximal urethra and the bladder. Both were normal. He has had no deterioration in his flow at short-term follow-up with no systemic manifestations of amyloidosis.
Figure 1: Histology with Congo Red stain.
Figure 2: Ascending urethrogram.
Discussion
The term amyloid means ‘‘starchlike’’ tissue deposits in the extracellular tissue that stained with iodine solutions. The principal constituent of the amyloid fibril is protein. Three pathogenetic mechanisms can cause the condition; genetic, immunogenic and chronic inflammatory. More than 25 proteins have been identified that may cause amyloidosis. Clinically however, the most important amyloidogenic proteins are serum amyloid A (SAA), transthyretin (TTR), and immunoglobulin kappa (IGK) or lambda light chains (IGL; so-called primary or AL type). These constitute more than 90% of systemic amyloidosis. It is commonly associated with rheumatoid arthritis, familial Mediterranean fever (Heredo-familial amyloidosis) and chronic haemodialysis [3]. Any organ within the urinary tract can be affected by amyloidosis. Renal amyloidosis is one of the commonest sites of systemic amyloidosis (AA). Primary amyloidosis of the lower ureter has previously been described presenting with ipsilateral hydronephrosis secondary to ureteric stricture and anuria as a result of bilateral ureteric involvement [4]. Amongst the lower urinary tract, the bladder is the most commonly affected site [5]. Both primary and secondary amyloidosis may involve the bladder. Painless visible hematuria is the main presenting symptoms in most (>75%) cases [6].
Urethral amyloidosis has been reported in men aged between 21 and 89 years. It can be associated with a variety of lower urinary tract symptoms which can be confused for a primary tumor of the urethra or nonspecific urethritis. It has been described in both sexes although only one case has been reported in a female [7]. Isolated amyloid of the penile urethra and corpus spongiosum have been reported, presenting with painful erections or haemospermia [8]. MRI may be useful in suggesting a diagnosis [9]. The early urethroscopic appearances of UA may resemble a simple fibrotic stricture but may subsequently develop a mass, which may ulcerate, cause pain or bleed, thereby raising concern of malignancy. Urethroscopy where possible, offers the best assessment of the urethra but one has to maintain a high index of suspicion as in our case. An intraoperative frozen section biopsy is essential to exclude a malignancy. One caveat of urethroscopic assessment is that if the lesion involves the corpus spongiosum it could be missed on urethroscopy as the pathological process does not involve the urethral mucosa. In this clinical setting either ultrasonography or preferably MRI imaging is required to demonstrate infiltrating lesion of the corpus spongiosum.
A variety of treatments have been described for the management of urethral amyloidosis ranging from urethral dilatation, transurethral resection, excision and perineal urethrostomy, anastomotic urethroplasty. a pedicle flap urethroplasty, a buccal mucosal graft urethroplasty and a penile skin graft. Evidence suggests that amyloidosis can affect the buccal mucosa and penile skin and hence it is possible that amyloid proteins may be deposited in the grafted tissue [10]. If recurrence of amyloidosis occurs in substituted tissue, time to recurrence may help guide the urethral surgeon as to the optimum secondary urethral procedure. As most cases have been managed differently, due to variation in clinical spectrum with no long term follow up, it is difficult to make generalized recommendations about treatment. At this point, we recommend that patients are best managed in a specialised unit with expertise in urethral surgery for best possible individualized care.
Conclusion
Amyloidosis of the lower urinary tract is unusual and has varied presentation requiring a case-by-case tailored approach in management of the disease. It is more common than expected in patients with a background of chronic inflammatory conditions. Thorough investigation is needed in a specialised unit to differentiate it from malignancy.
Acknowledgement
None.
Article Info
Article Type
Case ReportPublication history
Received: Sat 27, Mar 2021Accepted: Tue 17, Aug 2021
Published: Fri 03, Sep 2021
Copyright
© 2023 Sachin Malde. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2021.01.04
Author Info
Nawal Khan Kamran Ahmed Catherine Horsfield Sachin Malde Majed Shabbir
Corresponding Author
Sachin MaldeDepartment of Urology, Guy’s Hospital, London, UK
Figures & Tables
References
1.
Mitchell RN,
Kumar V, Cotran RS, Robbins SL (2003) Diseases of Immunity. Robb Bas Pathol
7: 103-164.
2.
Mangera A, Linton
KD, Fernando M, Channer J, Chapple CR (2012) What is the evidence for the
management of urethral amyloidosis? A systematic review of the literature. BJU
Int 109: 1858-1861. [Crossref]
3.
Gejyo F, Homma N,
Arakawa M (1993) Long-term complications of dialysis: pathogenic factors with
special reference to amyloidosis. Kidney Int Suppl 41: S78-S82. [Crossref]
4.
Kato Y, Sue Y,
Fujii H, Numata A, Yachiku S (2000) [Localized amyloidosis of the ureter and
bladder treated effectively by occlusive dressing technique therapy using
dimethyl sulfoxide: a case report]. Hinyokika Kiyo 46: 421-424. [Crossref]
5.
Livneh A, Shtrasburg
S, Martin BM, Baniel J, Gal R et al. (2001) Light chain amyloidosis of the
urinary bladder: A site restricted deposition of an externally produced
immunoglobulin. J Clin Pathol 54: 920-923. [Crossref]
6.
Johansson SL,
Cohen SM, Damjanov I, Linder J (1996) Lower urinary tract: Diseases of the
urogenital and reproductive systems. Ande Pathol 10: 2154.
7.
Kageyama S,
Suzuki K, Ushiyama T, Fujita K, Kawabe K (1998) Primary localized amyloidosis
of the urethra in a woman. Br J Urol 81: 918-919. [Crossref]
8.
Trívez Boned MA, Blas Marín M, García García MA, Gil
Martínez P, García de Jalón Martínez A et al. (2002) [Urethral amyloidosis]. Actas Urol Esp 26: 46-49. [Crossref]
9. Ichioka K, Utsunomiya N, Ueda N, Matsui Y, Yoshimura K et al. (2004) Primary localized amyloidosis of urethra: magnetic resonance imaging findings. Urology 64: 376-378. [Crossref]
10. Takahashi T, Miura H, Matsu ura Y, Iwana S, Maruyama R et al. (2005) Urine cytology of localized primary amyloidosis of the ureter: a case report. Acta Cytol 49: 319-322. [Crossref]
