Journals
Presurgical heterogeneity of lung 18FDG-PET uptake predicts acute exacerbation of interstitial lung disease following pulmonary resection in patients with smoke exposures
A B S T R A C T
Objectives: Acute exacerbation of interstitial lung disease (AEILD) is a severe complication after pulmonary resection. This study investigates whether 2-[18]-fluoro-2-deoxy-D-glucose (18F-FDG) uptake on positron emission tomography (PET) in ILD areas is a predictor of AEILD.
Methods: We enrolled 200 non-small cell lung cancer (NSCLC) patients with smoke exposures. Ten (5.0%) developed AEILD of whom seven were diagnosed as severe AEILD requiring medication. Patients were classified into either heterogeneity (HET, presence of ILD and non-ILD areas) or homogeneity (HOM, only non-ILD) according to the 18F-FDG uptake at diaphragm level.
Results: The average maximum 18F-FDG uptake by NSCLC and in non-ILD was in HOM comparable to that in HET (p = 0.86 and p = 0.14, respectively); however; it was 2.8-fold higher in ILD than in non-ILD within HET (p < 0.01). Multivariate analyses revealed that only HET was independent factors for AEILD and severe AEILD (p < 0.01, and p = 0.03, respectively). Among HET, honeycombing or a triad of reticulation, consolidation, and ground-glass attenuation on high-resolution computed tomography (n = 21) associated with higher complication rate regarding AEILD and worse prognosis (both p < 0.01).
Conclusions: Presurgical 18F-FDG uptake on normal lung areas may predict AEILD in smoke exposures.
Synopsis: Heterogeneity consisted of normal lung area and interstitial lung disease area according to 18F-FDG uptake. The average maximum 18F-FDG uptake was 2.8-fold higher in interstitial lung area than in normal area within heterogeneity. honeycombing or plenty of distinctive findings of interstitial lung disease on high-resolution computed tomography associated with higher complication rate regarding acute exacerbation of interstitial lung disease and worse prognosis.
Keywords
Positron emission tomography, non-small cell lung cancer, interstitial lung disease, pulmonary resection, honeycombing
Introduction
Lung cancer is a major cause of morbidity in the world [1, 2]. Lung cancer-related interstitial lung disease (ILD) is well known to be associated with idiopathic pulmonary fibrosis [3]. A recent review shows that the relative lung cancer risk is 3.5 to 7.3 times higher in ILD, with lung cancers occurrence estimated at 10–20% in ILD and more than 15% of patients with ILD likely dying from lung cancer [4]. The ILD incidence upon lung cancer diagnosis varies between 2.4% and 10.9% [4]. Cancer-related inflammation affects many aspects of the malignant process, including the proliferation and survival of malignant cells, angiogenesis, tumour metastasis, and tumour response to chemotherapeutic drugs and hormones [5]. In addition, ILD have an increased rate of cancer-related death after lung resection [6, 7]. Especially, the acute exacerbation of interstitial lung disease (AEILD) is currently left as the troublesome problem that should be still unsettled as complications after lung resection for lung cancers. In a Japanese dataset of 1,763 patients with resection of pulmonary non-small cell lung cancer (NSCLC), AEILD occurred in 9.3%, caused a mortality rate of 43.9%, and was with 71.7% the main cause for 30-day mortality [8, 9]. In addition, a different approach was that of Sato et al. who analysed in 2014 the 30-day risk of AEILD in a large-scale Japanese database [9]. The authors derived a risk score from AEILD history parameters, surgical procedures, the degree of elevated serum KL-6 levels, and the degree of vital capacity decreases [9]. However. enough verification has not been obtained because little is known to have focused on this risk score associated with AEILD after surgical resection.
Several studies describe that various physiological and radiological parameters can predict a poor outcome in patients with idiopathic pulmonary fibrosis (IPF). High-resolution computed tomography (HRCT) is a promising method to determine the extent of fibrosis [10]. The glucose uptake on 18F fluoro-deoxy-glucose positron emission tomography (18FDG-PET) has been suggested as a radiological method to identify poor survival in IPF patients in clinical use as biomarker [11, 12]. Groves et al. reported that an increased pulmonary 18F-FDG metabolism was observed in all patients with IPF and other forms of diffuse lung disease [13]. Moreover, recent reports present evidence that in IPF patients, a high 18F-FDG uptake is independently associated with an increased mortality risk. For instance, Umeda et al. demonstrate a strong association of 18F-FDG uptake with the patient’s prognosis [14].
Maniwa et al. suggest that 18F-FDG uptake may be a predictive factor for AEILD after surgery. They propose that surgeons should pay attention to 18F-FDG uptake in ILD areas when considering surgery for lung cancer patients with ILD [15]. In IPF patients, 18F-FDG uptake was also observed in lung areas with a normal morphological appearance on HRCT [16]. However, the prognostic value of FDG-PET after surgical resection of NLCLC remains unclear when the lung parenchyma appears normal in a HRCT? The aim of the present study was to evaluate whether 18F-FDG uptake in a pretreatment 18FDG-PET uptake may predict the risk of AEILD and the prognosis of NSCLC after pulmonary resection.
Materials and Methods
I Study design and patients
In this retrospective study, we examined 200 patients with primary NSCLCs, who had undergone pulmonary resection. All patients had a history of previous or current smoking. The pulmonary resections were performed between January 2010 and December 2016 at the Aichi Cancer Hospital. Data for the cancer classification were derived from computed tomography (CT) images of the chest and upper abdomen, magnetic resonance images of the head, and PET images. NSCLCs were staged using the TNM classification system (8th edition), and pathological diagnosis was interpreted according to the terminology outlined by the World Health Organization in 2004 [17, 18]. For this study, a CT consolidation on thin CT slices (10–20 mm thickness) served as a critical radiological measure. Data surveyed from patient records included age, sex, preoperative serum carcinoembryonic antigen levels, smoking status (pack-years), respiratory functions (vital capacity [VC], per cent forced expiratory volume in 1 second [FEV1%], and carbon monoxide diffusing capacity [DLCO]), pathological TNM stage, C-reactive protein (CRP) before and 3 months after pulmonary resection, mutation status (EGFR/KRAS/ALK), and AEILD during the follow-up course. AEILD was diagnosed by by pulmonologists. The PET images were re-examined if the HRCT of a patient indicated a potential AEILD. AEILD was diagnosed if an increase in 18F-FDG uptake was identified in lung areas that appeared normal in preoperative images. The AEILD cases (n = 10) were divided into two groups depending on having treatment (severe AEILD, n = 7) or not (n = 3). The interstitial pneumonia (IP) score was calculated according to the established formula from Japan [8].
This study was conducted in accordance with the Declaration of Helsinki. All patient records and information were anonymized prior to analysis. Since the anonymity of individual patients was ensured, the Institutional Review Board of Aichi Cancer Hospital approved this study (2016-1-208), provided that preoperative informed consent was obtained in each instance.
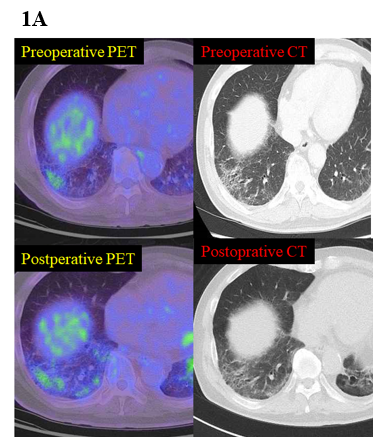
Figure 1: (A) A representative case of 18FDG-PET (right) and HRCT (left) before pulmonary resection (top) and at the time of an acute exacerbation of interstitial lung disease (bottom).
II PET-CT acquisition and distribution
All patients underwent 18FDG-PET prior to surgical resection. The generation and evaluation of 18FDG-PET scans has been described in detail in previously published articles [19-22]. PET-CT images were acquired according to a standard imaging protocol using a dedicated PET/CT scanner (Biograph 40; SIEMENS Healthcare Japan Co., Japan). Patients fasted for at least 6 hours prior to injection of 3.8 MBq/kg of 18F-FDG and were scanned 90 minutes after FDG injection. Parameters such as total lung glycolysis (TLG), as well as maximum and mean 18F-FDG uptake, were obtained by PET, whereas TLG was calculated as the product of mean 18F-FDG uptake and metabolic tumour volume. The maximum and mean 18F-FDG uptake and the metabolic tumour volume of NSCLC and ILD areas were calculated using a SIEMENS MMWP workstation, as previously reported [19-22]. A technical assistant blinded to the clinical data calculated these parameters. The difference in FDG uptake between both sides was evaluated by two radiologists. All patients were attributed to one of the following two groups according to their HRCT findings: heterogeneity (HET) consisting of ILD and non-ILD areas or homogeneity (HOM) presenting only non-ILD areas (Figure 1A).
III Statistical analyses
All data were calculated using standard software (SPSS version 25.0; SPSS Inc. [IBM Corporation], Chicago, IL, USA). Comparisons between two groups were performed using the Mann–Whitney U test. The Kaplan–Meier method was used to analyse survival rates in patient subsets; between-group differences in survival were assessed with the log-rank test. Potential correlates of survival were subjected to univariate and multivariate analyses using the Cox proportional hazards regression model. Hazard ratios (HRs) and median survival rates are presented with 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
Table 1: Patient characteristics
|
Variables |
HOM (n = 141) |
HET (n = 59) |
p value |
|
Sex male/female |
114/27 (80.9%) |
51/8 (86.4%) |
0.34 |
|
Age (years) |
67 |
71 |
<0.01* |
|
Pack-years (mean) |
45.5 ± 27.4 |
50.6 ± 25.9 |
0.01* |
|
Laboratory data |
|
|
|
|
CEA |
6.8 ± 13.1 |
6.7 ± 7.1 |
0.01* |
|
Preoperative CRP |
0.5 ± 1.2 |
0.5 ± 1.2 |
0.97 |
|
Postoperative CRP (3 months) |
0.9 ± 3.0 |
0.9 ± 1.9 |
0.14 |
|
Cancer location (right/left) |
92/49 (65.2%) |
32/27 (54.2%) |
0.14 |
|
Maximum 18FDG uptake |
|
|
|
|
Cancer |
13.7 ± 9.3 |
12.7 ± 7.6 |
0.86 |
|
Non-ILD area |
0.5 ± 0.2 |
0.5 ± 0.2 |
0.14 |
|
ILD area |
- |
1.4 ± 0.6 |
|
|
IP score |
|
|
|
|
Mean (range) |
6.1 ± 1.8 |
8.0 ± 2.4 |
<0.01* |
|
Respiratory function |
|
|
|
|
VC (%), mean (range) |
97.8 ± 14.3 |
96.0 ± 15.4 |
0.13 |
|
FEV1% (%), mean |
73.3 ± 11.1 |
74.8 ± 10.3 |
0.28 |
|
DLCO (%), mean |
101.3 ± 31.6 |
88.3 ± 22.0 |
<0.01* |
|
Pathology |
|
|
0.01 |
|
Adeno |
97 (68.8%) |
23 (40.0%) |
- |
|
Squamous |
27 (19.1%) |
30 (50.8%) |
- |
|
Others |
17 (12.1%) |
6 (10.2%) |
|
|
Pathological stage |
|
|
0.86 |
|
IA/IB |
63/31 (44.7/22.0%) |
26/12 (44.1/20.3%) |
- |
|
IIA/IIB |
1/26 (0.7/18.4%) |
2/9 (33.9/15.3%) |
- |
|
IIIA/IIB/IV |
18/1/1 (12.8/0.7/0.7%) |
10/0/0 (17.0/0/0%) |
- |
|
Mutations |
|
|
|
|
EGFR (positive) |
26 (18.4%) |
6 (10.1%) |
0.15 |
|
KRAS (positive) |
20 (14.2%) |
5 (8.4%) |
0.27 |
|
ALK (pos/neg/unknown) |
0/125/16 (0%) |
0/54/5 (0%) |
- |
|
Approach |
|
|
|
|
Thoracotomy/Thoracoscopy |
84/57 (59.6%) |
41/18 (69.5%) |
0.19 |
|
Procedure |
|
|
0.92 |
|
Sublobar |
49 (18.4%) |
8 (20.5%) |
|
|
Lobectomy |
216 (81.2%) |
31 (79.5%) |
|
|
Pneumonectomy |
1 (0.4%) |
0 (0%) |
|
18FDG: 2-[18]-fluoro-2-deoxy-D-glucose; ALK: ; CEA: carcinoembryonic antigen; CRP: C-reactive protein; DLCO: carbon monoxide diffusing capacity; EGFR: ; FEV1%: per cent forced expiratory volume in one second; HET: heterogeneity; HOM: homogeneity; ILD: interstitial lung disease; IP: interstitial pneumonia; KRAS: ; VC: vital capacity.
Results
I Patient characteristics
The clinicopathological characteristics of the study population are summarized in Table 1. The mean patient age was significantly higher in HET than in HOM (67 vs. 71 years, respectively, p < 0.01). Male participants accounted for at least 80% in both groups (p = 0.34). Exposure to tobacco as determined by pack-years was significantly higher in HET group compared to HOM (p < 0.01). Regarding respiratory function tests, VC and FEV1% were equivalent in both groups (p = 0.13 and p = 0.28, respectively); however, the mean DLCO value was significantly lower in HET compared to HOM (p < 0.01). The two groups did not show significant differences with respects to surgical approach and procedure (thoracotomy vs. thoracoscopy, p = 0.19; proportion of sublobar resection, p = 0.92). The proportion of adenocarcinoma was significantly more frequent in HOM than in HET (68.8% vs. 40.0%, respectively; p < 0.01); however, patients in HOM displayed an increased rate in pathological stages equal or higher than IIA (HOM 33.3% vs. HET 35.6%, respectively; p = 0.86). No significant between-group difference was observed with respect to mutations in EGFR and KRAS (p = 0.15 and p = 0.27). The IP risk score was 1.3-fold higher in HET than in HOM (p < 0.01).
II Accumulation of maximum 18FDG uptake on PET
The average maximum 18FDG uptake by primary lung cancer was in HOM as high as that in HET and did not show any statistically significant difference (p = 0.86). Comparing non-ILD areas, the maximum 18FDG uptake was not significantly different between HOM and HET (p = 0.14). By contrast, the average maximum 18FDG uptake was within HET 2.8-fold higher in ILD areas compared to non-ILD areas (p < 0.01).
III Acute exacerbations of interstitial lung disease
Ten patients (5.0%) presented an AEILD as a postoperative complication. Among those, seven patients (3.5%) required steroids (severe AEILD), whereas the remaining three (1.5%) recovered without further treatment after the radiological diagnosis. Only male patients experienced AEILDs. Compared with HOM, HET had a 21.9-fold higher complication rate regarding AEILDs (0.7% vs. 15.3%, p < 0.01) and a 14.6-fold higher complication rate regarding severe AEILDs (0.7% vs. 10.2%, p = 0.01). Univariate analyses determined four variables, including patient age ≧ 75 years, postoperative inflammatory procrastination (elevated CRP levels for 3 months), the presence of HET, and an IP score > 10, that were used in subsequent multivariate analyses. As shown in (Table 2), postoperative inflammatory procrastination and HET were independent factors for AEILD (both p < 0.01). Likewise, the presence of HET was the only factor independently associated with severe AEILD (p = 0.03).
Table 2: Univariate and multivariate analyses of clinicopathologic variables associated with an acute exacerbation of interstitial lung disease
|
Variables |
Uni- |
Multivariate |
||||
|
|
|
|
AEILD |
Severe AEILD |
||
|
|
|
p |
HR (CI) |
p |
HR (CI) |
p |
|
Patient characteristics |
|
|
|
|
|
|
|
|
Age ≧75 years |
0.04 |
3.76 (0.76–18.6) |
0.11 |
1.95 (0.38–10.1) |
0.42 |
|
|
Pack-year >60 |
0.50 |
- |
- |
|
|
|
FEV1 (%) |
|
|
|
|
|
|
|
|
FEV1 <70 |
0.91 |
- |
- |
|
|
|
Laboratory CRP |
|
|
|
|
|
|
|
|
Preoperative inflammation (>1.0) |
0.43 |
- |
- |
|
|
|
|
Postoperative (> 1.0) |
<0.01 |
15.9 (2.9–85.8) |
<0.01 |
4.29 (0.82–22.5) |
0.09 |
|
Intraoperative findings |
|
|
|
|
|
|
|
|
Time (>240 min) |
0.75 |
- |
- |
|
|
|
PET |
|
|
|
|
|
|
|
|
HET |
<0.01 |
25.8 (2.68–249.2) |
<0.01 |
12.9 (1.34–119.8) |
0.03 |
|
Procedures |
|
|
|
|
|
|
|
|
Non-sublobar |
0.89 |
- |
|
|
|
|
Approach |
|
|
|
|
|
|
|
|
Thoracoscopy |
0.62 |
- |
|
|
|
|
IP score |
|
|
|
|
|
|
|
|
IP score (>10) |
0.01 |
2.45 (0.39–15.6) |
0.34 |
1.85 (0.28–12.2) |
0.53 |
|
Genomic mutation |
|
|
|
|
|
|
|
|
EGFR |
0.99 |
- |
- |
|
|
AEILD: acute exacerbation of interstitial lung disease; CI: confidence interval; CRP: C-reactive protein; EGFR: Epidermal growth factor receptor; FEV1: forced expiratory volume in one second; HET: heterogeneity; HR: hazard ratio; IP: interstitial pneumonia; PET: positron emission tomography.
IV Parenchymal patterns of ILD areas on HRCT and PET
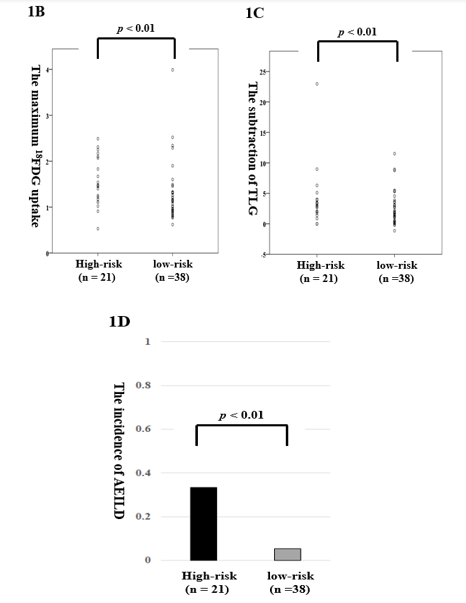
In HET, parenchymal patterns on HRCT consisted of honeycombing (n = 12, 20.3%), reticulation (n = 36, 61.0%), consolidation (n = 32, 52.4%), and ground-glass attenuation (GGA, n = 50, 84.7%). We subdivided the HET patients into two groups: a high-risk group with either honeycombing or a triad containing all elements of reticulation, consolidation, and GGA (n = 21) and a low-risk group, in which one or two elements of the defined triad were present (n = 38). The average maximum 18FDG uptake in ILD areas was 1.2-fold higher in the high-risk group (1.55 ± 0.52) than in the low-risk group (1.27 ± 0.64, p = 0.01; Fig. 1B). The mean TLG which subtracted that in non-ILD area from ILD area was equivalent with no significant (p = 0.08; Fig. 1C). Moreover, compared to the low-risk group, the high-risk group had a 21.9-fold higher complication rate regarding AEILDs (33.3% vs. 5.3%, p < 0.01; Figure 1D).
Figure 1: (B/C) The levels of the maximum 18FDG uptake (B) and TLG (C) according to HRCT status in heterogeneity. (D) AEILD incidence according to HRCT status in heterogeneity.
V Patient outcomes
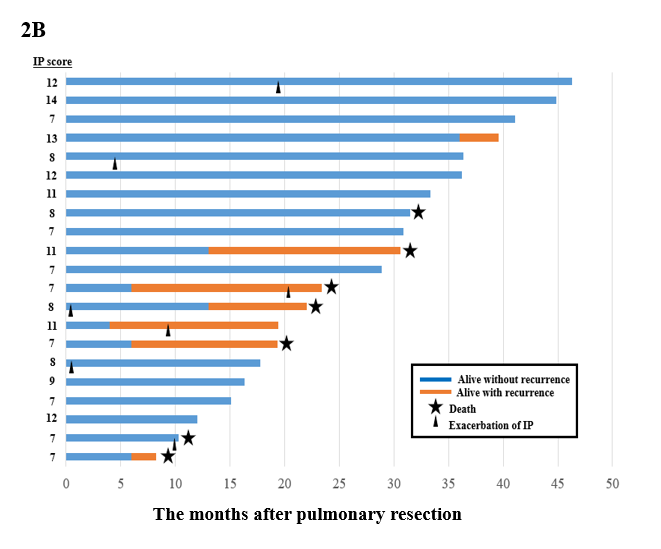
The median follow-up after pulmonary resection was 29.0 months (range, 4.4-99 months). No significant difference in overall survival was observed between low-risk group in HET and HOM (HR: 0.63, 95%CI: 0.22–1.83, p = 0.40), but overall survival was significantly lower in high-risk group in HET compared to HOM (HR: 2.50, 95%CI: 1.05–5.91, p = 0.04; Figure 2A). As shown in Figure 2B, of the 21 HET patients in the high-risk group, 7 presented a cancer recurrence, 5 died due to cancer-related causes, and 2 died from cardiopulmonary events (Figure 2B).
Figure 2: (A) Overall survival from pulmonary resection according to homogeneity, heterogeneity (high-risk and low-risk divided by HRCT) on PET-CT scans. (B) A schematic representation of the postoperative course.
Discussion
In the present study, we investigated the relationship between 18FDG metabolism on PET and the occurrence of AEILDs during the postoperative period and retrospectively analysed on HRCT appearance patterns in ILD areas to establish prognostic markers. The main findings of this study can be summarized as follows: 1) by dividing diversity accumulation on PET, patients from HET had a 21.9- and 14.6-fold higher complication risks for AEILD and severe AEILD, respectively, compared to those from HOM; 2) multivariate analyses established that HET was an independent factor for both AEILD and severe AEILD; and 3) based on a more than two-year follow-up period, our study revealed that specific parenchymal patterns on HRCT define a high-risk subgroup in HET which is significantly associated with a worse overall survival compared to the low-risk in HET and HOM.
In NSCLC, 18FDG-PET has recently become indispensable for reliable diagnostic imaging, and several surgical studies demonstrated the prognostic significance of pretreatment 18FDG uptake [23, 24]. We previously reported that a high 18FDG uptake is associated with tumour invasiveness and lymph node metastases in NSCLCs [20]. Recently, several authors suggested that 18FDG-PET might be useful as an index for IPF activity, and Umeda et al. reported in 2015 that positive 18FDG-PET findings are associated with overall and progression-free survival in patients with IPF [11-14]. Subsequently, Win et al. described that IPF patients can have increased 18FDG-PET uptake even in lung areas with normal morphological appearance on HRCT [16]. In another study, the same authors investigated the consistency of pulmonary 18FDG-PET uptake in patients with IPF and found an excellent short-term (6.3 ± 4.3 days) reproducibility [25]. The present study revealed that the significant difference in 18FDG-PET uptake in non-ILD areas between HOM and HET, but the mean maximum 18FDG-PET uptake was 2.8-fold higher in ILD areas compared with non-ILD areas.
It is well-known that increased 18FDG-PET uptake may not only be an indicator of malignancy but also inflammation. Many authors published that AEILD after pulmonary resection results in a poorer prognosis because of a refractory respiratory failure. However, only a few studies investigated the relationship between AEILD and preoperative 18FDG-PET uptake. In 2013, Maniwa et al. reported that the presurgical 18FDG-PET uptake may be a predictive factor for AEILD after pulmonary resection [15]. Similar to these results, our study demonstrates that 18FDG-PET uptake in the HET group is an independent predictive factor for both AEILD and severe AEILD. The suitability of maximum 18FDG uptake as a predictive factor for AEILD after pulmonary resection remains unclear; however; most studies demonstrate a significant association between maximum 18FDG uptake and IPF [11-14]. Several authors proposed the mechanism of 18FDG-PET uptake in ILD areas in vitro and in vivo as follows: (a) in ILD, a stimulation of inflammatory cells induces an overexpression of the glucose transporter-1; (b) the infiltration of inflammatory cells causes an increase in activated fibroblast foci; and (c) 18FDG-PET uptake in HRCT-identified ILD areas as well as unaffected regions of the lung parenchyma reflects the increase in inflammatory cells [26]. In another study from 2016, Jacquelin et al. suggested that 18FDG-PET could assess the reversibility of interstitial lung infiltrates by predicting the functional improvement and, therefore, could help physicians to predict and monitor treatment response [27]. Our results suggest that postoperative inflammatory procrastination was one of the independent factors for AEILD, which represents infiltration by inflammatory cell and activated fibroblast foci. However, further studies are necessary to establish the relationship between preoperative 18FDG-PET uptake and AEILD because only a small retrospective cohort was used in this study.
In another part of this study, we postulated that 18FDG-PET uptake corresponds to morphological characteristics of ILD areas. The HET high-risk group (honeycombing or triad of reticulation, consolidation, and GGA) was indeed associated with a poorer prognosis after an NSCLC was treated with pulmonary resection. Several authors describe that honeycombing and reticulation on HRCT are associated with microscopic fibrotic changes, which might promote an increase in 18FDG-PET uptake [11, 13, 16]. In 25 consecutive IPF patients, Win et al. first provide evidence that PET can detect abnormalities in the absence of changes in lung parenchyma on HRCT [16]. In another study, a high pulmonary target-to-background ratio is independently associated with increased risk of mortality in IPF patients [11]. Groves et al. reported that the parenchymal pattern on HRCT at the maximal 18FDG-PET was GGA in 19.4%, reticulation/honeycombs in 72.2%, and mixed 8.3% in 36 IPF and maximal 18FDG-PET uptake corresponded to areas of reticulation/honeycombing on HRCT [13]. The distribution of 18FDG-PET uptake corresponded to the parenchymal abnormalities, representing the existence of irreversible microscopic fibrosis. In the present study, the HET group with a higher 18FDG-PET uptake in ILD areas was a predictive factor for both AEILD and severe AEILD, and parenchymal patterns in the HET group presenting honeycombing or the entire triad of reticulation, consolidation, and GGA carried a higher risk for both AEILD and severe AEILD than the remaining patterns. This study has several limitations. First, our surgical population has a relatively low proportion of patients with mild chronic obstructive pulmonary disease as previously reported [28]. The accuracy in the diagnosis of AEILD data consistency is believed to be improved by additional PET evaluation in a follow-up course, which is our strong asset in this study. In this study, severe AEILD occurred in 10.2% of patients in the HET group, which is compatible with comparable Japanese data [7-9]. Second, our study revealed only a small number of AEILDs, possibly due to the retrospective, single-centre design, which may have caused bias. Although further investigations will be required, we believed this cohort study is an invaluable asset because there are few reports about the relationship between 18FDG-PET uptake and AEILD. Third, in PET studies, measurements deviate among facilities. It is a strong point of this cohort study that the PET measurements were performed at a single institutional PET facility as described in our previous report [20]. However, future accumulations, particularly for the planning phase, are still necessary for validating our results.
In conclusion, the presurgical 18F-FDG uptake on normal lung area predicted AEILD in patients with the smoke exposure. In addition, patients with a heterogenous ILD area who presented on HRCT honeycombing or the triad of reticulation, consolidation, and GGA had a higher AEILD complication rate and a worse 3-year overall survival rate. Additional studies are warranted to determine if pretreatment 18F-FDG uptake is associated with the occurrence of AEILD and the patient’s prognosis.
Acknowledgements
None.
Conflict of Interest
All authors declare that there is no conflict of interest.
Author Contributions
Conception and design: HK, NS, YT and TH. Data collection: HK, SY, and KN, Radiological interpretation: SY, and IH, Data analysis and interpretation: HK, NS, and YO. Writing of the manuscript: HK. Supervise of this manuscript: YS, and TH.
Disclosures and Funding Information
No funding was declared.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 28, May 2019Accepted: Thu 13, Jun 2019
Published: Tue 09, Jul 2019
Copyright
© 2023 Hiroaki Kuroda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2019.02.08
Author Info
Yuko Oya Hiroaki Kuroda Hiroshi Iwata Keita Nakanishi Noriaki Sakakura Toyoaki Hida Yukinori Sakao Yusuke Sugita Yusuke Takahashi
Corresponding Author
Hiroaki KurodaDepartment of Thoracic Surgery, Aichi Cancer Center
Figures & Tables
Table 1: Patient characteristics
|
Variables |
HOM (n = 141) |
HET (n = 59) |
p value |
|
Sex male/female |
114/27 (80.9%) |
51/8 (86.4%) |
0.34 |
|
Age (years) |
67 |
71 |
<0.01* |
|
Pack-years (mean) |
45.5 ± 27.4 |
50.6 ± 25.9 |
0.01* |
|
Laboratory data |
|
|
|
|
CEA |
6.8 ± 13.1 |
6.7 ± 7.1 |
0.01* |
|
Preoperative CRP |
0.5 ± 1.2 |
0.5 ± 1.2 |
0.97 |
|
Postoperative CRP (3 months) |
0.9 ± 3.0 |
0.9 ± 1.9 |
0.14 |
|
Cancer location (right/left) |
92/49 (65.2%) |
32/27 (54.2%) |
0.14 |
|
Maximum 18FDG uptake |
|
|
|
|
Cancer |
13.7 ± 9.3 |
12.7 ± 7.6 |
0.86 |
|
Non-ILD area |
0.5 ± 0.2 |
0.5 ± 0.2 |
0.14 |
|
ILD area |
- |
1.4 ± 0.6 |
|
|
IP score |
|
|
|
|
Mean (range) |
6.1 ± 1.8 |
8.0 ± 2.4 |
<0.01* |
|
Respiratory function |
|
|
|
|
VC (%), mean (range) |
97.8 ± 14.3 |
96.0 ± 15.4 |
0.13 |
|
FEV1% (%), mean |
73.3 ± 11.1 |
74.8 ± 10.3 |
0.28 |
|
DLCO (%), mean |
101.3 ± 31.6 |
88.3 ± 22.0 |
<0.01* |
|
Pathology |
|
|
0.01 |
|
Adeno |
97 (68.8%) |
23 (40.0%) |
- |
|
Squamous |
27 (19.1%) |
30 (50.8%) |
- |
|
Others |
17 (12.1%) |
6 (10.2%) |
|
|
Pathological stage |
|
|
0.86 |
|
IA/IB |
63/31 (44.7/22.0%) |
26/12 (44.1/20.3%) |
- |
|
IIA/IIB |
1/26 (0.7/18.4%) |
2/9 (33.9/15.3%) |
- |
|
IIIA/IIB/IV |
18/1/1 (12.8/0.7/0.7%) |
10/0/0 (17.0/0/0%) |
- |
|
Mutations |
|
|
|
|
EGFR (positive) |
26 (18.4%) |
6 (10.1%) |
0.15 |
|
KRAS (positive) |
20 (14.2%) |
5 (8.4%) |
0.27 |
|
ALK (pos/neg/unknown) |
0/125/16 (0%) |
0/54/5 (0%) |
- |
|
Approach |
|
|
|
|
Thoracotomy/Thoracoscopy |
84/57 (59.6%) |
41/18 (69.5%) |
0.19 |
|
Procedure |
|
|
0.92 |
|
Sublobar |
49 (18.4%) |
8 (20.5%) |
|
|
Lobectomy |
216 (81.2%) |
31 (79.5%) |
|
|
Pneumonectomy |
1 (0.4%) |
0 (0%) |
|
18FDG: 2-[18]-fluoro-2-deoxy-D-glucose; ALK: ; CEA: carcinoembryonic antigen; CRP: C-reactive protein; DLCO: carbon monoxide diffusing capacity; EGFR: ; FEV1%: per cent forced expiratory volume in one second; HET: heterogeneity; HOM: homogeneity; ILD: interstitial lung disease; IP: interstitial pneumonia; KRAS: ; VC: vital capacity.
Table 2: Univariate and multivariate analyses of clinicopathologic variables associated with an acute exacerbation of interstitial lung disease
|
Variables |
Uni- |
Multivariate |
||||
|
|
|
|
AEILD |
Severe AEILD |
||
|
|
|
p |
HR (CI) |
p |
HR (CI) |
p |
|
Patient characteristics |
|
|
|
|
|
|
|
|
Age ≧75 years |
0.04 |
3.76 (0.76–18.6) |
0.11 |
1.95 (0.38–10.1) |
0.42 |
|
|
Pack-year >60 |
0.50 |
- |
- |
|
|
|
FEV1 (%) |
|
|
|
|
|
|
|
|
FEV1 <70 |
0.91 |
- |
- |
|
|
|
Laboratory CRP |
|
|
|
|
|
|
|
|
Preoperative inflammation (>1.0) |
0.43 |
- |
- |
|
|
|
|
Postoperative (> 1.0) |
<0.01 |
15.9 (2.9–85.8) |
<0.01 |
4.29 (0.82–22.5) |
0.09 |
|
Intraoperative findings |
|
|
|
|
|
|
|
|
Time (>240 min) |
0.75 |
- |
- |
|
|
|
PET |
|
|
|
|
|
|
|
|
HET |
<0.01 |
25.8 (2.68–249.2) |
<0.01 |
12.9 (1.34–119.8) |
0.03 |
|
Procedures |
|
|
|
|
|
|
|
|
Non-sublobar |
0.89 |
- |
|
|
|
|
Approach |
|
|
|
|
|
|
|
|
Thoracoscopy |
0.62 |
- |
|
|
|
|
IP score |
|
|
|
|
|
|
|
|
IP score (>10) |
0.01 |
2.45 (0.39–15.6) |
0.34 |
1.85 (0.28–12.2) |
0.53 |
|
Genomic mutation |
|
|
|
|
|
|
|
|
EGFR |
0.99 |
- |
- |
|
|
AEILD: acute exacerbation of interstitial lung disease; CI: confidence interval; CRP: C-reactive protein; EGFR: Epidermal growth factor receptor; FEV1: forced expiratory volume in one second; HET: heterogeneity; HR: hazard ratio; IP: interstitial pneumonia; PET: positron emission tomography.

References
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69: 7-34. [Crossref]
- Sakakura N, Mizuno T, Kuroda H, Arimura T, Yatabe Y et al. (2018) The eighth TNM classification system for lung cancer: A consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer 118: 134-138. [Crossref]
- Liu Y, Zhu M, Geng J, Ban C, Zhang S et al. (2018) Incidence and radiologic-pathological features of lung cancer in idiopathic pulmonary fibrosis. Clin Respir J 12: 1700-1705. [Crossref]
- Naccache JM, Gibiot Q, Monnet I, Antoine M, Wislez M et al. (2018) Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 10: 3829-3844. [Crossref]
- Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C et al. (2017) Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 8: 103117-103128. [Crossref]
- Kumar P, Goldstraw P, Yamada K, Nicholson AG, Wells AU et al. (2003) Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 125: 1321-1327. [Crossref]
- Chida M, Kobayashi S, Karube Y, Hayama M, Tamura M et al. (2012) Incidence of acute exacerbation of interstitial pneumonia in operated lung cancer: institutional report and review. Ann Thorac Cardiovasc Surg 18: 314-317. [Crossref]
- Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M et al. (2014) Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 147: 1604-1611. [Crossref]
- Sato T, Kondo H, Watanabe A, Niwa H, Horio H et al. (2015) A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 63: 164-172. [Crossref]
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ et al. (2018) Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198: e44-e68. [Crossref]
- Thomeer M, Grutters JC, Wuyts WA, Willems S, Demedts MG (2010) Clinical use of biomarkers of survival in pulmonary fibrosis. Respir Res 11: 89. [Crossref]
- Win T, Screaton NJ, Porter JC, Ganeshan B, Maher TM et al. (2018) Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging 45: 806-815. [Crossref]
- Groves AM, Win T, Screaton NJ, Berovic M, Endozo R et al. (2009) Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med 50: 538-545. [Crossref]
- Umeda Y, Demura Y, Morikawa M, Anzai M, Kadowaki M et al. (2015) Prognostic Value of Dual-Time-Point 18F-FDG PET for Idiopathic Pulmonary Fibrosis. J Nucl Med 56: 1869-1875. [Crossref]
- Maniwa T, Endo M, Isaka M, Nakagawa K, Ohde Y et al. (2014) Acute exacerbation of interstit ial lung disease with lung cancer after surgery: evaluation with 2-[18]-fluoro-2-deoxy-D-glucose positron emission tomography. Surg Today 44: 494-498. [Crossref]
- Win T, Thomas BA, Lambrou T, Hutton BF, Poter JC et al. (2014) Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging 41: 337-342. [Crossref]
- Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F et al. (2016) The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 11: 1204-1223. [Crossref]
- Beasley MB, Brambilla E, Travis WD (2005) The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 40: 90-97. [Crossref]
- Dejima H, Kuroda H, Oya Y, Sakakura N, Inaba Y et al. (2018) Evaluation of lobar lymph node metastasis in non-small cell lung carcinoma using modified total lesion glycolysis. J Thorac Dis 10: 6932-6941. [Crossref]
- Hiroaki Kuroda, Shnsuke Mori, Hirotaka Tanaka, Tatsuya Yoshida et al. (2018) Prognostic significance of combined radiologic imaging modalities for prognosis of clinical IA adenocarcinomas. Oncotarget 9:10745-10753. [Crossref]
- Seto K, Kuroda H, Yoshida T, Sakata S, Mizuno T et al. (2018) Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag Res 10: 2117-2124. [Crossref]
- Yoshida T, Tanaka H, Kuroda H, Shimizu J, Horio Y et al. (2016) Standardized uptake value on (18)F-FDG-PET/CT is a predictor of EGFR T790M mutation status in patients with acquired resistance to EGFR-TKIs. Lung Cancer 100: 14-19. [Crossref]
- Madsen PH, Holdgaard PC, Christensen JB, Høilund-Carlsen PF (2016) Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging 43: 2084-2097. [Crossref]
- Kirienko M, Cozzi L, Antunovic L, Lozza L, Fogliata A et al. (2018) Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging 45: 207-217. [Crossref]
- Win T, Lambrou T, Hutton BF, Kayani I, Screaton NJ et al. (2012) 18F-Fluorodeoxyglucose positron emission tomography pulmonary imaging in idiopathic pulmonary fibrosis is reproducible: implications for future clinical trials. Eur J Nucl Med Mol Imaging 39: 521-528. [Crossref]
- El-Chemaly S, Malide D, Yao J, Nathan SD, Rosas IO et al. (2013) Glucose transporter-1 distribution in fibrotic lung disease: association with [¹⁸F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest 143: 1685-1691. [Crossref]
- Jacquelin V, Mekinian A, Brillet PY, Nunes H, Fain O et al. (2016) FDG-PET/CT in the prediction of pulmonary function improvement in nonspecific interstitial pneumonia. A Pilot Study. Eur J Radiol 85: 2200-2205. [Crossref]
- Iizuka S, Kuroda H, Yoshimura K, Dejima H, Seto K et al. (2016) Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 8: 985-991. [Crossref]
