Journals
Predictors of cognitive anosognosia in older adults with suspected dementia
A B S T R A C T
Objective: Anosognosia heterogeneously affects cognitive functioning and indeed, at the very onset of dementia symptoms. This raises the question of accountable predictors of each cognitive impairment. The objective of this study was to identify the cognitive and non-cognitive predictors of so-called cognitive anosognosia.
Method: A paradigm based on the discrepancies between performance predictions and actual performance in the light of the Dementia Rating Scale (DRS) helped distinguish cognitive anosognosia in relation to four major functions: Attention Initiation-perseveration, Conceptualisation and Memory. Patients achieved a complete set of neuropsychological tests and assessments of the level of anxiety, apathy and functional independence.
Results: Significant correlations existed between all cognitive anosognosia scores (AS) and neuropsychological performance scores of the participants. Similarly, significant correlations were found between the Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scores and the Initiation and Conceptualisation AS and between anxiety score and Conceptualisation and Memory AS. However, the regression models were exclusively cognitive for Attention Initiation-perseveration and Memory AS. Only the Conceptualisation AS was predicted by a non-strictly cognitive regression model including the ADL score.
Conclusions: If the different specific cognitive anosognosias are predicted by separate sets of variables, our results emphasise that dysexecutive impairment plays a major role regardless of the deficiencies considered.
K E Y W O R D S
Anosognosia, cognitive disorders, dementia, affects, ADL
Introduction
Public significance statements: The predictors of anosognosia are primarily cognitive. The predictors vary depending on the “object” of awareness. The affects do not have any impact on cognitive anosognosia. Executive impairment is the main predictor of cognitive anosognosia.
Cognitive disorders occur with advancing age, and anosognosia is a condition that can compromise diagnoses and/or acceptance of support. Anosognosia is defined as the alteration of “the reasonable or realistic perception or appraisal of a given aspect of one’s situation, functioning or performance, or of the resulting implications” [1].
During the prodromal stage, upon suspicion of cognitive impairment, despite a lower intensity and frequency than in the diagnosed diseases, anosognosia is already a factor associated with poor prognosis [2-4].
This symptom is considered a direct consequence of the disease and heterogeneously affects cognitive functions, such as hardly affecting memory processes at the onset of the disease and visuo-constructive abilities or executive functions at a later stage [5-8].
This heterogeneity in the objects of awareness raises the question of identifying the general or specific anosognosia predictors for each cognitive impairment [9]. In dementia, the first hypotheses explored the involvement of memory disorders while many current studies report correlations between anosognosia and neuropsychological processes such as executive processes, in particular [10-14].
The Cognitive Awareness Model provides an understanding of anosognosia as resulting from a deficiency in the modules that, under executive control, regulate our behaviors and recognize mistakes via comparators, which compare current performance with prior experiences (stored in a Personal DataBase) [15]. In anosognosia, information updates are no longer assured, errors are no longer taken into account, and behavior is therefore not adjusted to match current performance. According to the modules involved, deficiencies involve separate initial disorders and various expressions of anosognosia [5, 15].
Research on predictors of awareness of cognitive deficiencies –cognitive anosognosia –faces several challenges at the moment of the suspicion of dementia disorder.
Most researches measure generally little awareness of cognitive disorders (e.g., the functional intelligence score of the Anosognosia Questionnaire for Dementia (AQ-D) [16, 17]. In line with the CAM model, we should study the diversity of cognitive processes globally or specifically involved in the awareness of various cognitive deficiencies.
In addition, other non-cognitive clinical factors can contribute to the awareness of disorders and of behavioral disorders in particular, including apathy anxiety, and difficulties in daily life activities [18-21].
The objective of this study was to identify cognitive and non-cognitive predictors of cognitive anosognosia. We expected to find specific cognitive and non-cognitive predictors for each score measuring the awareness of disorders. This study involved patients with signs of early impairment or existing undiagnosed cognitive impairment. A paradigm based on the discrepancies between actual performance and performance predictions helps to distinguish the awareness of cognitive disorders, function by function.
Method
Participants
Patients were recruited from a Memory Center in the Bateliers Hospital in Lille, France. The entire protocol was performed over one-half of a day, in accordance with a standard Memory Center consultation. Patients were submitted to a complete clinical and neuropsychological evaluation, after which a probable diagnosis was made based on published criteria.
The inclusion criteria included suspicion of cognitive impairment, a Mini-Mental State Examination greater than or equal to 15 points and fluency in French. Another criterion was the presence of an informing caregiver from the patient's personal life, hence not a professional [22]. The participants were then asked to complete the Functional Independence questionnaires in a hetero-evaluation, as in a conventional evaluation situation. The criteria for non-inclusion included the presence of uncorrected sensory deficiencies that might prevent the realization of a classical clinical picture, the presence of a language disorder such as aphasia, or a major deficiency in the understanding and diagnosis of a psychotic table (Axis1 DSM-IV).
Consent from each patient and family caregiver was collected prior to any evaluation, in accordance with the Declaration of Helsinki.
Cognitive and psychological assessment
The MMSE and the Dementia Rating Scale were used as measures of overall cognitive functioning [22, 23]. The DRS has allowed us to characterize the five major cognitive functions: attention, initiation-perseveration, construction, conceptualization and memory.
The memory assessment was performed using the Buschke Selective Reminding Test (immediate recall, total free and total recall, delayed total and free recall, and recognition) [24]. Visuospatial abilities were evaluated by copying the figures of the Consortium to Establish a Registry for Alzheimer's Disease [25]. The tests of executive functions measured mental flexibility inhibition (Victoria Stroop Test (VST), perseverative errors (Buschke test, TMT B, VST uncorrected errors) and verbal initiation (literal fluency - letter P) [26-28]. Some patients did not have the ability to perform all the tests because of major cognitive disorders.
To the above assessments, we added a functional independence and affective hetero evaluation by a caregiver. Functional independence was assessed using the following scales: The Activities of Daily Living and Instrumental Activities of Daily Living scales [29, 30]. We investigated the anxiety level using the Hamilton Anxiety Depression Scale, retaining only the items concerning anxiety [31]. Apathy was studied through the total score of the Apathy Inventory [32].
Measurement of anosognosia
The Multidimensional Isomorphic Simple Awareness (MISA) procedure was used to assess cognitive anosognosia among the participants (for additional details, see Antoine et al., 2013) [6]. This tool is based on subjective self-ratings that are compared with actual objective performance on neuropsychological tests. The patients were asked to predict their DRS performance on a dichotomous scale for each of the tasks of the DRS after hearing and seeing the task and prior to actually performing the task. Each patient was required to simply predict whether he/she would perform the task (1) well or (2) incorrectly before performing each main item of the DRS. From the predictions and the real score (RS), a prediction score (PS) is calculated for each subscale. This PS represents the quantity of correct answers that a participant believes he will obtain for each item presented. If a participant overestimates his own performance, his PS will be significantly higher than his real performance (i.e., the RS). For the anosognosia score (AS), we calculated the discrepancy between the RS and the newly calculated PS.
This procedure was applied to 4 of the 5 subscales of the DRS: attention, initiation-perseveration, conceptualization and memory. The construction subscale was not included because of the small number of items in that subscale, which would not allow a reliable comparison with other subscales.
Design of the scores
The DRS exhibits an inconvenience in its subscales in that the total scores differ, which makes their comparison awkward when added together. To provide each subscale the same importance in the DRS overall score, we used the weighted mean for the RS rather than for the PS. Thus, we corrected each subscale score by their respective number of items and converted it into a percentage to facilitate readability. For example, the total score for the attention subscale was divided by 37, which corresponds to the maximum score for this subscale and then multiplied by 100 to obtain a percentage. Finally, all the corrected subscale scores were averaged to obtain the weighted mean for the overall DRS score for the RS and the PS. All analyses were conducted with all scores in terms of the percentage of good answers.
Statistical analyses
We used a Pearson’s correlation coefficient to observe the links between the scores of anosognosia (AS) by category and the results for different neuropsychological tests. We conducted bivariate and multiple stepwise regression analysis on the specific AS to highlight the neuropsychological predictors for each category. Each variable was included for a probability of less than 0.01 and excluded for a probability greater than 0.05. The final regression model chosen corresponds to the significant model with the highest adjusted R².
Results
Seventy-six patients met the set of criteria and therefore exhibited a suspicion of cognitive disorders. Their demographic and clinical data are summarized in Table 1.
Table 1: Patients’ clinical and demographic data
|
Gender (women, %) |
71.05% |
|
|
Age (±SD) |
82.4 (6.1) |
|
|
MMSE |
23.8 (3.5) |
|
|
DRS Total RS weighted mean |
84.7 (9.5) |
|
|
DRS Total AS weighted mean |
8.2 (6.3) |
|
|
Attention AS |
n=76 |
5.9 (4.7) |
|
Initiation AS |
n=76 |
8.5 (10.0) |
|
Conceptualization AS |
n=76 |
5.2 (7.2) |
|
Memory AS |
n=76 |
15.4 (14.6) |
Note. MMSE, Mini Mental Test Examination; DRS, Dementia Rating Scale; RS, Real Score; AS, Anosognosia Score.
Correlations
A bivariate correlation matrix is presented in Table 2 and was performed using 4 cognitive ASs (attention, initiation-perseveration, conceptualization, memory) and neuropsychological, affective and functional independence variables.
Table 2: Correlations between DRS-specific ASs (Attention, Initiation-Perseveration, Conceptualization, and Memory) with neuropsychological tests and affective and functional independence questionnaires
|
Correlations between specific ASs and neuropsychological and functional data |
||||
|
|
Attention AS |
Initiation AS |
Concept AS |
Memory AS |
|
Buschke test |
|
|
|
|
|
Immediate recall |
- |
-0.35** |
-0.27* |
-0.48*** |
|
Total free recall |
- |
-0.40*** |
- |
-0.26* |
|
Total total recall |
- |
-0.36** |
- |
- |
|
Delayed free recall |
- |
-0.36** |
- |
- |
|
Delayed total recall |
- |
-0.38** |
- |
- |
|
Recognition |
- |
-0.28* |
- |
-0.36** |
|
Intrusions |
- |
- |
- |
- |
|
Perseverative errors |
- |
- |
0.50*** |
0.58*** |
|
CERAD figures |
- |
-0.32** |
0.39*** |
0.24* |
|
Trail Making Test Reaction time |
0.32* |
- |
- |
- |
|
Trail Making Test Perseverative errors |
0.46** |
- |
- |
- |
|
Victoria Stroop Test Reaction time |
|
- |
- |
- |
|
Victoria Stroop Test Inhibition errors |
0.33** |
0.49*** |
0.42*** |
- |
|
“P” Alphabetic fluency |
0.32** |
0.45*** |
0.42*** |
- |
|
Total ADL |
- |
-0.43*** |
-0.43*** |
- |
|
Total IADL |
- |
-0.34** |
-0.31** |
- |
|
HADS – Anxiety |
- |
- |
0.25* |
0.24* |
|
Total IA |
- |
- |
- |
- |
Note. DRS, Dementia Rating Scale; AS, Anosognosia Score. ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; IA, Apathy Inventory; Pearson’s correlation coefficient; *p<0.05; **p<0.01; ***p<0.001. The AS of the attention subscale was positively correlated with 3 tests: TMT (reaction time: r=0.32, p<0.05, PE: r=0.46, p<0.01), VST (IE: r=0.33, p<0.01)) and alphabetical fluency (r=0.32, p<0.01). However, attention AS was not correlated with the total score of the HADS (r=0.07, p=0.5705), IA (r=-0.19, p=0.11), ADL (r=-0.15, p=0.224) or IADL (r=-0.08, p=0.498).
Initiation AS correlated with all subscores of the Buschke test (except for PE, all p<0.05), the test of the CERAD (r=-0.32, p<0.01), VST (IE: r=0.49, p<0.001) and alphabetical fluency (r=0.45, p<0.001) as well as with the total scores of the ADL (r=-0.43, p<0.001) and IADL (r=-0.34, p<0.01). Initiation AS was not correlated with other measures (HADS, r=0.07, p=0.55; IA, r=0.09, p=0.47).
Conceptualization AS showed significant correlations with the Buschke test (ImmR: r=-0.27, p<0.05; PE: r=0.50, p<0.001), the test of the CERAD (r=0.39, p<0.001), the VST (IE: r=0.42, p<0.001) and alphabetical fluency(r=0.42, p<0.001)as well as with the total scores of the HADS (r=0.25, p<0.05), ADL (r=-0.43, p<0.001) and IADL (r=-0.31, p<0.01).Only the IA was not correlated (r=0.06, p=0.65).
Finally, Memory AS was significantly correlated to the Buschke test (ImmR: r=-0.48, p<0.001; Total free recall: r=-0.26, p<0.05; Recognition: r=-0.36, p<0.01; PE: r=0.58, p<0.001) and with the test of the figures of the CERAD (r=0.24, p<0.05), as well as with the HADS anxiety score (r=0.2425, p<0.05). Memory AS was not correlated with the total scores in the IA (r=0.01, p=0.94), ADL (r=-0.11, p=0.389) and IADL (r=-0.12, p=0.343).
Stepwise regression analyses
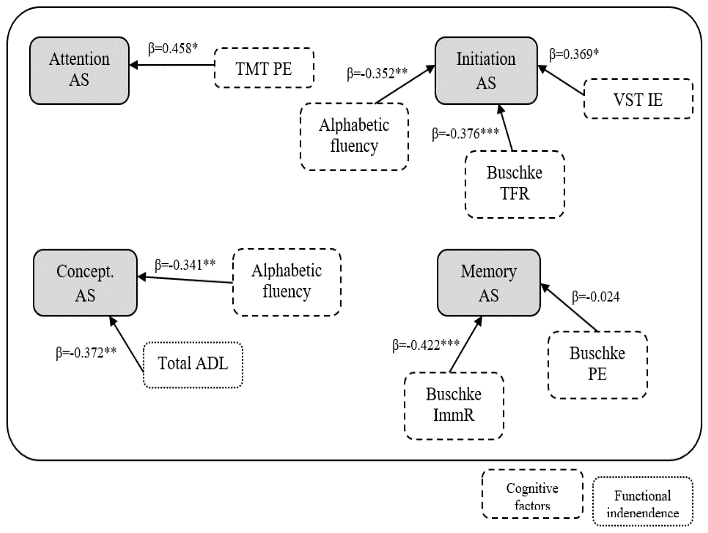
Regression analyses following the stepwise method were used to determine the links between AS (a single dependent variable) and cognitive, affective and independence disorders (as predictor variables). Table 3 contains each step for all 4 scores of anosognosia, and Figure 1 summarizes the significant predictive models by AS.
Figure 1: Modeling of specific AS predictors
Table 3: Stepwise regression analyses
|
|
||
|
N=41 |
AR² |
β |
|
Step 1 |
0.189 |
|
|
TMT B-A Perseverative errors |
|
0.458** |
|
Initiation – Perseveration AS |
|
|
|
N=63 |
AR² |
β |
|
Step 1 |
0.229 |
|
|
Victoria Stroop Inhibition errors |
|
0.492*** |
|
Step 2 |
0.336 |
|
|
Victoria Stroop Inhibition errors |
|
0.399** |
|
“P” Alphabetic fluency |
|
-0.328** |
|
Step 3 |
0.490 |
|
|
Victoria Stroop Inhibition errors |
|
0.369** |
|
“P” Alphabetic fluency |
|
-0.352** |
|
Total Free recall Buschke Test |
|
-0.376*** |
|
Conceptualization AS |
|
|
|
N=66 |
AR² |
β |
|
Step 1 |
0.173 |
|
|
Total ADL |
|
-0.430*** |
|
Step 2 |
0.278 |
|
|
Total ADL |
|
-0.372** |
|
“P” Alphabetic fluency |
|
-0.341** |
|
Memory AS |
|
|
|
N=69 |
AR² |
β |
|
Step 1 |
0.332 |
|
|
Buschke Perseverative errors |
|
-0.014 |
|
Step 2 |
0.323 |
|
|
Buschke Perseverative errors |
|
-0.024 |
|
Recognition Buschke Test |
|
-0.422*** |
Note. AS, Anosognosia Score. AR², Adjusted R²; TMT, Trail Making Test; ADL, Activities of Daily Living; *p<0.05; **p<0.01; ***p<0.001.
Only the attention AS was predicted by a single variable: TMT PE (F=10.33, p<0.01, R2=0.189, β=0.458, p<0.01).
The AS scores of conceptualization and memory were explained by the regression models with two variables, respectively; total ADL (β=-0.37, p<0.01) – alphabetical fluency (β=-0.34, p<0.01) (F=12.15, p<0.01, R2=0.278) and immediate recall (β=-0.42, p<0.001) – PE Buschke (β=-0.02, p=0.82) (F=18.63, p<0.001, R2=0.323).
The score of anosognosia on the initiation-perseveration subscale was predicted by a model with 3 predictors as follows: VSTIE (β=-0.37, p<0.01) – alphabetical fluency (β=-0.35, p<0.01) – total free recall Buschke (β=-0.38, p<0.001) (F=17.30, p<0.001, R2=0.489).
Discussion
The objective of this study was to identify cognitive and non-cognitive predictors of anosognosia and their relation to the disturbances of different cognitive functions. We expected to find specific predictors for each awareness disorders score and to identify cognitive and non-cognitive predictors.
Neuropsychological and non-cognitive actors of cognitive anosognosia
Our results initially confirmed significant correlations between the cognitive ASs and neuropsychological performance scores of the participants. We also observed significant correlations between the loss of functional independence score and the ASs of initiation and conceptualization. An equivalent significant link was found between the anxiety score and awareness disorders in conceptualization and memory.
Clinical correlations of cognitive anosognosia are not restricted to cognitive functioning. Other dimensions of the patient's life are considered, including functional independence and its affects, which correlates with the current literature [16]. However, we did not find any significant link between cognitive anosognosia and the presence of apathy, although this result has been previously widely documented [18].
Each of the four cognitive ASs correlated significantly with a specific set of cognitive and non-cognitive variables. Therefore, it appears that cognitive anosognosia should not be considered as a whole but instead divided into "specific cognitive anosognosias" for each deficiency, i.e., specific to each “object “of awareness [9]. This design has already been noted in studies considering anosognosia as a set of heterogeneous phenomena [7, 21]. Furthermore, based on observed correlations, we have emphasized a regression model for each cognitive impairment considered.
Predictors of cognitive anosognosia
The second important result showed that the predictors of different ASs differed from one another. The various specific cognitive anosognosias are predicted by separate sets of variables, which reinforce the heterogeneous nature of anosognosia and demonstrates that each object of awareness has a different origin. Therefore, anosognosia must be assessed at a process level to better understand this phenomenon.
In addition, although the correlations showed many links between all considered clinical variables and the four scores in which anosognosia was measured, the regression models are exclusively cognitive for attention, initiation-perseveration and memory ASs. Only the conceptualization AS is predicted by a model that is not focused strictly on cognitive decline and includes the total ADL score. Furthermore, deficiencies that predict the best ASs relate either mostly to the executive processes or the memory processes. Indeed, out of seven predictors that were identified, five are related to the executive domain.
Specifically, the results show the involvement of specific executive processes that are perseverative errors and predictors of three ASs out of four. The perseverations are signs that are characteristic of executive impairment and show a deficiency in terms of monitoring and updating online information [33].
These results are consistent with the CAM model of Morris and Mograbi (2013) for which the comparator mechanisms are at two levels: sensory-motor and central cognitive [15]. This last level is underpinned by attentional, executive (monitoring) and mnemonic (semantic memory) networks that are typically affected by neurodegenerative diseases. Thus, the entanglement of executive disorders that prevent the updating of information and memory disorders that disrupt the encoding and retrieval form a solid basis for the emergence of anosognosia. The type of anosognosia should vary based on the disorders and therefore the cognitive modules that are affected, which our study seems to support.
Cognitive anosognosia and daily life
Our study tends to confirm that daily activities have a significant impact on anosognosia in terms of cognitive deficiencies. However, if the ADLs are negatively and significantly correlated with scores of anosognosia, then these findings only pertain to the executive scales of conceptualization and initiation-perseveration and not to those scales involving memory processes. In regression models, the ADLs are only predictors of the conceptualization AS, a scale corresponding to high level executive processes. The ADLs are known to be affected by deficiencies in executive functions, and their involvement in the perception of cognitive impairment also seems now confirmed.
This study has several limitations. Analyses were limited by the size of the total sample and by the diagnostic category in particular. It would be interesting to pursue this type of research for each type of diagnosis, such as Alzheimer's disease, MCI, and vascular and mixed dementia. Furthermore, the anosognosia assessment methodology could be improved. The methodology in this study is based on a scale screening of cognitive functions whose scores remain composite. A study involving deficiencies that were less coarse would continue the modeling work of anosognosia.
Conclusions
Despite these limitations, our results emphasize that executive dysfunction plays a major role in cognitive anosognosia and associated deficiencies in memory, attention, initiation-perseveration and conceptualization. Executive functioning involves very different processes, and the challenge is how to identify those that are more involved. In addition, some predictors are attributable to other areas, including mnemonic [16]. The multiplicity of the phenomena might suggest the involvement of additional variables not considered in our study. Future studies are required to more comprehensively identify the phenomena involved and to study their roles and relative weights in structural modeling processes of anosognosia.
Funding and grants
This work was supported by the Conseil Départemental du Nord and the Médéric Alzheimer Foundation and was developed through LabEx (excellence laboratory, program investment for the future), DISTALZ (Development of Innovative Strategies for a Transdisciplinary Approach to ALZheimer Disease) and the MESHS (Maison Européenne des Sciences de l’Homme et de la Société, Lille, France).
Article Info
Article Type
Research ArticlePublication history
Received: Wed 13, Jun 2018Accepted: Wed 27, Jun 2018
Published: Fri 17, Aug 2018
Copyright
© 2023 Emilie Avondino. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.PDR.2018.02.001
Author Info
Dominique Huvent-Grelle Emilie Avondino François Puisieux Jean Roche Pascal Antoine
Corresponding Author
Emilie AvondinoUniversité de Lille, SCALab, CNRS UMR 9193, France
Figures & Tables
Table 1: Patients’ clinical and demographic data
|
Gender (women, %) |
71.05% |
|
|
Age (±SD) |
82.4 (6.1) |
|
|
MMSE |
23.8 (3.5) |
|
|
DRS Total RS weighted mean |
84.7 (9.5) |
|
|
DRS Total AS weighted mean |
8.2 (6.3) |
|
|
Attention AS |
n=76 |
5.9 (4.7) |
|
Initiation AS |
n=76 |
8.5 (10.0) |
|
Conceptualization AS |
n=76 |
5.2 (7.2) |
|
Memory AS |
n=76 |
15.4 (14.6) |
Table 2: Correlations between DRS-specific ASs (Attention, Initiation-Perseveration, Conceptualization, and Memory) with neuropsychological tests and affective and functional independence questionnaires
|
Correlations between specific ASs and neuropsychological and functional data |
||||
|
|
Attention AS |
Initiation AS |
Concept AS |
Memory AS |
|
Buschke test |
|
|
|
|
|
Immediate recall |
- |
-0.35** |
-0.27* |
-0.48*** |
|
Total free recall |
- |
-0.40*** |
- |
-0.26* |
|
Total total recall |
- |
-0.36** |
- |
- |
|
Delayed free recall |
- |
-0.36** |
- |
- |
|
Delayed total recall |
- |
-0.38** |
- |
- |
|
Recognition |
- |
-0.28* |
- |
-0.36** |
|
Intrusions |
- |
- |
- |
- |
|
Perseverative errors |
- |
- |
0.50*** |
0.58*** |
|
CERAD figures |
- |
-0.32** |
0.39*** |
0.24* |
|
Trail Making Test Reaction time |
0.32* |
- |
- |
- |
|
Trail Making Test Perseverative errors |
0.46** |
- |
- |
- |
|
Victoria Stroop Test Reaction time |
|
- |
- |
- |
|
Victoria Stroop Test Inhibition errors |
0.33** |
0.49*** |
0.42*** |
- |
|
“P” Alphabetic fluency |
0.32** |
0.45*** |
0.42*** |
- |
|
Total ADL |
- |
-0.43*** |
-0.43*** |
- |
|
Total IADL |
- |
-0.34** |
-0.31** |
- |
|
HADS – Anxiety |
- |
- |
0.25* |
0.24* |
|
Total IA |
- |
- |
- |
- |
Table 3: Stepwise regression analyses
|
|
||
|
N=41 |
AR² |
β |
|
Step 1 |
0.189 |
|
|
TMT B-A Perseverative errors |
|
0.458** |
|
Initiation – Perseveration AS |
|
|
|
N=63 |
AR² |
β |
|
Step 1 |
0.229 |
|
|
Victoria Stroop Inhibition errors |
|
0.492*** |
|
Step 2 |
0.336 |
|
|
Victoria Stroop Inhibition errors |
|
0.399** |
|
“P” Alphabetic fluency |
|
-0.328** |
|
Step 3 |
0.490 |
|
|
Victoria Stroop Inhibition errors |
|
0.369** |
|
“P” Alphabetic fluency |
|
-0.352** |
|
Total Free recall Buschke Test |
|
-0.376*** |
|
Conceptualization AS |
|
|
|
N=66 |
AR² |
β |
|
Step 1 |
0.173 |
|
|
Total ADL |
|
-0.430*** |
|
Step 2 |
0.278 |
|
|
Total ADL |
|
-0.372** |
|
“P” Alphabetic fluency |
|
-0.341** |
|
Memory AS |
|
|
|
N=69 |
AR² |
β |
|
Step 1 |
0.332 |
|
|
Buschke Perseverative errors |
|
-0.014 |
|
Step 2 |
0.323 |
|
|
Buschke Perseverative errors |
|
-0.024 |
|
Recognition Buschke Test |
|
-0.422*** |
References
1. Clare L, Rowlands J, Bruce E, Surr C, Downs M (2008) ‘I don't do like I used to do’: A grounded theory approach to conceptualising awareness in people with moderate to severe dementia living in long-term care. Soc Sci Med 66: 2366-2377. [Crossref]
2. Kalbe E, Salmon E, Perani D, Holthoff V, Sorbi S, et al. (2005) Anosognosia in very mild Alzheimer’s disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord 19: 349-356. [Crossref]
3. Orfei MD, Varsi AE, Blundo C, Celia E, Casini AR, et al. (2010). Anosognosia in mild cognitive impairment and mild Alzheimer's disease: Frequency and neuropsychological correlates. Am J Geriatr Psychiatry 18: 1133-1140. [Crossref]
4. Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, et al. (2002) Functional deficits in patients with mild cognitive impairment: Prediction of AD. Neurology 58: 758-764. [Crossref]
5. Hannesdottir K, Morris RG (2007) Primary and secondary anosognosia for memory impairment in patients with Alzheimer's disease. Cortex 43: 1020-1030. [Crossref]
6. Antoine P1, Nandrino JL, Billiet C (2013) Awareness of deficits in Alzheimer's disease patients: Analysis of performance prediction discrepancies. Psychiatry Clin Neurosci 67: 237-244. [Crossref]
7. Avondino E, Antoine P (2016) Heterogeneity of cognitive anosognosia and its variation with the severity of dementia in patients with Alzheimer’s disease. J Alzheimers Dis 50 : 89-99. [Crossref]
8.Marková IS, Clare L, Wang M, Romero B, Kenny G (2005) Awareness in dementia: Conceptual issues. Aging Ment Health 9: 386-393. [Crossref]
9. Marková IS, Clare L, Whitaker CJ, Roth I, Nelis SM, et al. (2014) Phenomena of awareness in dementia: Heterogeneity and its implications. Conscious Cogn 25: 17-26. [Crossref]
10. McGlynn SM, Schacter DL (1989) Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol 11: 143-205. [Crossref]
11. Antonella De Carolis, Virginia Cipollini, Valentina Corigliano, Anna Comparelli, Micaela Sepe-Monti, et al. (2015) Anosognosia in people with cognitive impairment: Association with cognitive deficits and behavioral disturbances. Dement Geriatr Cogn Dis Extra 5: 42-50. [Crossref]
12. Amanzio M, Vase L, Leotta D, Miceli R, Palermo S, et al. (2013) Impaired awareness of deficits in Alzheimer's disease: The role of everyday executive dysfunction. J Int Neuropsychol Soc 19: 63-72. [Crossref]
13. Gambina G, Bonazzi A, Valbusa V, Condoleo M, Bortolami O, et al. (2014) Awareness of cognitive deficits and clinical competence in mild to moderate Alzheimer’s disease: Their relevance in clinical practice. Neuro Sci 35: 385-390. [Crossref]
14. Satler C, Tomaz C (2013) Cognitive anosognosia and behavioral changes in probable Alzheimer’s disease patients. Dementia Neuropsychologia 7: 197-205.
15. Morris RG, Mograbi DC (2013) Anosognosia, autobiographical memory and self-knowledge in Alzheimer's disease. Cortex 49: 1553-1565.
16. Starkstein SE (2014). Anosognosia in Alzheimer's disease: Diagnosis, frequency, mechanism and clinical correlates. Cortex 61: 64-73. [Crossref]
17. Starkstein SE, Sabe L, Chemerinski E, Jason L, Leiguarda R (1996) Two domains of anosognosia in Alzheimer's disease. J Neurol Neurosurg Psychiatry 61: 485-490. [Crossref]
18. Starkstein SE, Jorge R, Mizrahi R, Robinson RG (2006). A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77: 8-11. [Crossref]
19. Clare L, Nelis SM, Martyr A, Roberts J, Whitaker CJ, et al. (2012) The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: Testing a biopsychosocial model. Int J Geriatr Psychiatry 27: 167-177. [Crossref]
20. Conde-Sala JL, Reñé-Ramírez R, Turró-Garriga O, Gascón-Bayarri J, Juncadella-Puig M, et al. (2013) Clinical differences in patients with Alzheimer’s disease according to the presence or absence of anosognosia: Implications for perceived quality of life. J Alzheimers Dis 33: 1105-1116. [Crossref]
21. Dourado MC, Mograbi DC, Santos RL, Sousa MF, Nogueira ML, et al. (2014) Awareness of disease in dementia: Factor structure of the assessment scale of psychosocial impact of the diagnosis of dementia. J Alzheimers Dis 41: 947-956. [Crossref]
22. Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198. [Crossref]
23. Mattis S (1976) Mental status examination for organic mental syndrome in the elderly patient. Geriatric Psychiatry 11: e121.
24. Van der Linden M, Adam S (2004). L'évaluation des troubles de la mémoire : Présentation de quatre tests de mémoire épisodique (avec leur étalonnage). Groupe de Boeck.
25. Morris JC, Edland S, Clark C, Galasko D, Koss E, et al. (1993) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology 43: 2457-2465. [Crossref]
26. Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills 8: 271-276.
27. Bayard S, Erkes J, Moroni C, Collège des Psychologues Cliniciens spécialisés en Neuropsychologie du Languedoc Roussillon (CPCN Languedoc Roussillon) (2011) Victoria Stroop Test: Normative data in a sample group of older people and the study of their clinical applications in the assessment of inhibition in Alzheimer's disease. Arch Clin Neuropsychol 26 : 653-661. [Crossref]
28. Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y (1990) Evocation lexicale formelle et sémantique chez des sujets normaux. Performances et dynamiques de production en fonction du sexe, de l'âge et du niveau d'étude [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. Acta Neurol Belg 90: 207-217. [Crossref]
29. Lawton MP, Brody EM (1969) Assessment of older people: Self-maintaining and instrumental activities of daily Living. Gerontologist 9: 179-186. [Crossref]
30. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914-919. [Crossref]
31. Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370. [Crossref]
32. Brocker P, Clairet S, Benoit M, Robert PH (2003) Inventaire Apathie : Évaluation de l’apathie chez des sujets présentant une maladie d’Alzheimer ou un trouble cognitif léger. [Apathy Inventory: Assessment of apathy on people with Alzheimer’s disease or mild cognitive impairment]. Revue de Gériatrie 28 : 473-480.
33. Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, et al. Groupe de Réflexion sur l'Evaluation des Fonctions Exécutives Study Group (2010) Dysexecutive syndrome: Diagnostic criteria and validation study. Ann Neurol 68: 855-864. [Crossref]
