Journals
Platelet-Rich Fibrin: A Versatile Purpose for Alveolar Ridge Preservation
A B S T R A C T
Clinical use of platelet-rich fibrin (PRF) have incresead in dental procedures and represents a promising alternative in the regeneration of soft and hard tissues, whether associated with biomaterials or alone. PRF presentation forms in membrane, plug or sticky bone are discussed. Versatility, biological security of autologous material, simplicity, cost-effectiveness and maleability are attractive factors to the multivariate use of PRF in-office. However, more randomized clinical trials protocol standardization are required to obtain reproducible results. The choice of most indicated therapeutic modality of PRF applied to alveolar ridge preservation should be made based on evidence to ensure a good clinical results in-office and patient satisfaction.
Introduction
The maintenance of the alveolar ridge after the tooth extraction is essential from the aesthetic and functional point of view, to ensure the appropriate tissue condition for implant and oral rehabilitation therapy [1, 2]. The local blood supply decreases with a dental and periodontal loss and there is more accelerated bone atrophy, especially of the thin buccal wall [1]. To avoid this common incident in dental practice, techniques of guided bone regeneration are recommended, using resorbable membranes and bone grafts of autogenous, allogenic, xenogeneic or alloplastic origin [3, 4]. The use in Brazil of bone substitute biomaterials in the filling of dental alveoli may exceed a quarter of the cases of tooth extraction in private clinics, demonstrating good cost-effectiveness and good professional acceptance [3]. However, in the brazilian public health system, there is a great demand for edentulism and a chronic underfunding, which motivates the search for regenerative strategies less dependent on commercial scaffolds to guarantee bone preservation [4].
Brief Literature Review
Since the first use of platelet-rich fibrin (PRF) in France reported in 2001 by Choukron, the reports in the last 18 years in the clinical use of PRF have increased [5]. PRF is a functional autologous fibrin matrix obtained by immediate centrifugation of the blood immediately collected from patient without the use of additives by the own dentist in-office [6, 7]. Although the technique of preparation, standing time, transfer process, temperature of centrifuge and vibration are factors for the mixed results reported in the literature, PRF represents a promising alternative in the regeneration of soft and hard tissues, whether associated with biomaterials or alone [2, 7, 8].
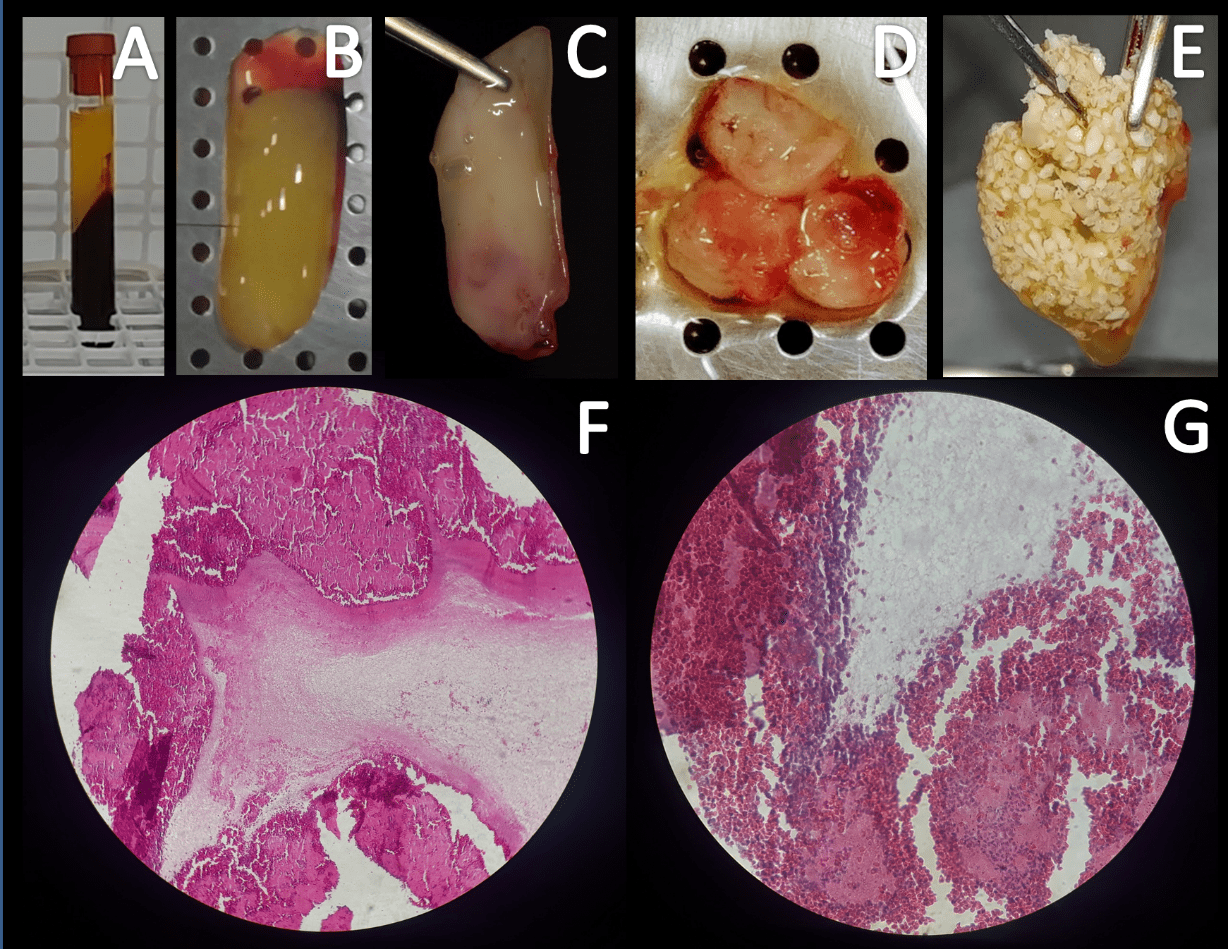
The biological bases of PRF reside in the high concentration of fibrin, platelets and leukocytes, which stimulates cell agglutination and growth factors involved with angiogenesis, the repair mechanisms, the immunological and antibacterial action, reducing surgical sequelae of pain and infection [8]. PRF may be present as a covering membrane of the dental alveolus, plug of filling the tooth socket or in the hybrid form of fibrin gel with fragments of bone graft or sticky bone (Figure 1) [9-11].
PRF membrane exhibits clinical and radiographic findings that are like the collagen membrane when used as a coating of freeze-dried bone allograft in grade II furcation defects in molars in up to 6 months of follow-up, reinforcing their contributive role in guided bone regeneration and representing an alternative to other expensive membranes [9].
Socket plug technique with or without the use of plaster of Paris as bone substitute in alveolar ridge preservation contributes to better post-operative healing and minimal loss of alveolar width and height for long term [10]. As for the autologous concentrated growth factors (CGF), they enriched bone graft matrix (sticky bone) and CGF-enriched fibrin membrane provides stabilization of bone graft in the defect, and therefore, accelerates tissue healing, bone maturation and minimizes bone loss during healing period [11].
Figure 1: After centrifugation (A) human PRF can be isolated (B). PRF in the form of a membrane (C), plug (D) or sticky bone (PRF+biphasic calcium phosphate, E). Histological images of fibrin-like material and intense cellularity (F, 100x, HE) compound by platelets, leukocytes and erythrocytes (G, 400x, HE). Images kindly provided by Ponte JS and Castro-Silva II in reference to own research registered in Brazil using PRF (CEP-UVA, CAAE n. 91602218.0.0000.5053, protocol n. 2.806.761).
A bibliographic survey of nine selected articles in the last 10 years showed that PRF presents effective and safe use when used in combination with biomaterials or isolated to accelerate the healing of soft and hard tissues, in addition to presenting low risk associated with satisfactory clinical results. Disadvantages in the use of PRF were associated to its protocol for obtention including handling, blood collection time and its transference for the centrifuge. A minimal experience of clinician for PRF manipulation can reduce all disadvantages listed before [5].
A systematic review (SR) about efficacy of PRF in bone regeneration of the jaws demonstred which its use appears to improve the local conditions of the grafts and soft tissues, reducing the healing times and symptoms. Four randomized clinical trials (RCT) in maxillary sinus lift surgery have not shown any difference using PRF in comparison to the traditional technique, 2 RCTs indicated that PRF in combination with autogenous bone improves bone volume and 3 articles evaluated alveolar preservation (1 RCT and 2 SRs) demonstrating that there may be an increase in the density of neoformed bone in the long term with a lower rate of buccal/lingual reabsorption [12].
A SR and meta-analysis of regenerative potential of PRF in intra-bony defects and furcation defects was carried out based on 24 RCTs. Significant pocket depth reduction, clinical attachment level gain and bone fill (mean value of 1.7 ± 0.7 mm) was achieved when comparing PRF to open flap debridement in intra-bony defects and furcation defects [13]. Simplicity, cost-effectiveness, and user-friendliness/malleability are attractive factors to the multivariate use of PRF [7]. However, more randomized clinical trials protocol standardization are required to obtain reproducible results [12, 13].
Conclusion
The choice of most indicated therapeutic modality of PRF applied to alveolar ridge preservation should be made based on evidence to ensure a good clinical results in-office and patient satisfaction.
Acknowledgments
Our special thanks to Coordination for the Improvement of Higher Education Personnel (CAPES-DS and PROAP-UFC-PPGB grants, Brazil) and Ceara State Foundation of Support for Scientific and Technological Development (FUNCAP, process #BP3-0139-00270.01.00/18, Brazil) for the financial support.
Article Info
Article Type
Short CommunicationPublication history
Received: Mon 17, Jun 2019Accepted: Mon 01, Jul 2019
Published: Sat 06, Jul 2019
Copyright
© 2023 Igor Iuco Castro da Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2019.03.05
Author Info
Igor Iuco Castro da Silva Jose Sandro Ponte Lana Karine Araújo Maria Alcineide Dias Araújo
Corresponding Author
Igor Iuco Castro da SilvaPostgraduate Program in Biotechnology, Federal University of Ceara (UFC), Avenida Maurocelio Rocha Pontes 100, 62042-280, Sobral (CE), Brazil
Figures & Tables
References
- Du Toit J, Siebold A, Dreyer A, Gluckman H (2016) Choukroun Platelet-Rich Fibrin as an Autogenous Graft Biomaterial in Preimplant Surgery: Results of a Preliminary Randomized, Human Histomorphometric, Split-Mouth Study. Int J Periodontics Restorative Dent 36: S75-S86. [Crossref]
- Amit Arvind Agrawal (2017) Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases 5: 159-171. [Crossref]
- Castro-Silva II, Coutinho LACR (2013) Use of bone grafts in Dentistry: profile of dentists from Niteroi/RJ. Rev bras odontol 69: 154-158.
- Castro-Silva II, Lima FMS, Granjeiro JM (2013) Bone grafts in Brazilian Dentistry: overview, challenges and prospects on the vision of Health Management. Rev flum odontol 39: 63-71.
- Andrade LS, Leite LP, Melo-Silva FB, Brito-Resende RF, Guedes-de-Uzeda MJ (2018) The use of platelet-rich fibrin concentrate in tissue healing and regeneration in dentistry. Int J Growth Factors Stem Cells Dent 1: 23-26.
- Castro-Silva II (2018) Platelet-rich fibrin for bone tissue regeneration. Otorhinolaryngol Head Neck Surg 3: 1-1.
- Zumarán CC, Parra MV, Olate SA, Fernández EG, Muñoz FT et al. (2018) The 3 R's for Platelet-Rich Fibrin: A "Super" Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials (Basel) 11: E1293. [Crossref]
- Choukroun J, Diss A, Simonpieri A, Girard MO, SChoeffler C et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101: 56-60. [Crossref]
- Mehta DB, Deshpande NC, Dandekar SA (2018) Comparative evaluation of platelet-rich fibrin membrane and collagen membrane along with demineralized freeze-dried bone allograft in Grade II furcation defects: A randomized controlled study. J Indian Soc Periodontol 22: 322-327. [Crossref]
- Girish-Kumar N, Chaudhary R, Kumar I, Arora SS, Kumar N et al. (2018) To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation: a prospective randomised controlled study. Oral Maxillofac Surg 22: 135-142. [Crossref]
- Sohn D-S, Huang B, Kim J, Park WE, Park CC (2015) Utilization of Autologous Concentrated Growth Factors (CGF) Enriched Bone Graft Matrix (Sticky Bone) and CGF-Enriched Fibrin Membrane in Implant Dentistry. JIACD 7: 11-29.
- Millard JL, Apablaza EM, Sirandoni RF, Carmona AV, Diaz DP (2018) Efficacy of Platelet-Rich Fibrin in Bone Regeneration of the Jaws: A Systematic Review. Res Rep Oral Maxillofac Surg 2: 007.
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P et al. (2017) Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intrabony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 44: 67-82. [Crossref]
