Phytochemical Screening and In Vivo Anti-Inflammatory Activities of Anti-Cancer Plant: Rutidea parviflora (Rubiaceae)
A B S T R A C T
The rapid development of malignant cancers is characterized by inflammation, which poses a significant drawback in cancer therapy. Both cancer and inflammation operate on very similar mechanisms involving angiogenesis and cell proliferation. Currently, cancer-intrinsic inflammations have been shown to promote cancer progression and hinder apoptosis of cancerous cells. Thus, an effective strategy for chemoprevention and therapy would involve the control of inflammation. This research work aims to investigate the anti-inflammatory activity of the extracts of the root bark of Rutidea parviflora (Rubiaceae), a plant I previously reported for anti-ovarian cancer activities and the isolation of palmatine; an anti-cancer compound and a second compound; urs-12-ene-24-oic acid, 3-oxo, methyl ester. This plant is renowned for its anti-inflammatory properties amongst locals in Delta state, Nigeria, which has necessitated this present research. Organic and aqueous extracts were obtained from the pulverized root bark by use of the America national cancer institute protocol (NCI). The organic extract was partitioned sequentially in increasing order of polarity with n-hexane, ethyl acetate, n-butanol and distilled water to obtain four fractions. Phytochemical screening was done using standard procedures. Results from the phytochemical screening indicated the presence of alkaloids, flavonoids, saponins, tannins, glycosides and carbohydrates. Anti-inflammatory investigations of the extracts and fractions were carried out by the induction of inflammation. The animals were grouped into 12 test groups and 2 control groups with 6 rats per group. Egg albumin (0.1 ml) was administered sub-plantarly followed by treatment. Group A received a dose of 200 mg/kg of the plant extracts and Group B received a dose of 400 mg/kg of the plant extracts. Group C (positive control) received indomethacin (10 mg/kg), while Group D (negative control) received 1 ml of normal saline. Statistical analysis showed significance against the negative control indicated by P<0.05 for extracts and fractions. While for the fourth hour post induction of inflammation; the activities of the Group B organic extract, ethyl acetate and n-butanol fractions were comparable with indomethacin indicating that the plant possess significant anti-inflammatory activity and warrants further anti-inflammatory studies.
Keywords
Rutidea parviflora, phytochemical, anti-inflammatory activity, acute inflammation
Introduction
As far back as 1863, Rudolf Virchow observed a link between cancer and inflammation [1]. Future researches have shown that both diseases are characterized by enhanced cell proliferation, survival, migration and angiogenesis. Chronic inflammation has been implicated in 25% of cancers. Cancer of the pancreas and prostate were mostly initiated by inflammations of these organs. In particular, chronic ulcerating colitis and Crohn’s disease are significant contributory factors to the development of colorectal cancer [2]. While acute inflammation may have been beneficial in the treatment of squamous cancer of the bladder [3]. This is not often the case as the presence of acute inflammation could lead to chronic inflammation, thus paving the way for cancer formation.
The presence of mutated cells and inflammatory mediators are characteristic of most tumor inflammations; a signal that both cancer and inflammation may be connected by the intrinsic and extrinsic pathways [4, 5]. Given that numerous mechanisms are associated with inflammations in the cancer progression process, the need for proper elucidation becomes paramount for novel therapy development. Considering the fact that inflammation and cancer have similar pathways, it follows that by targeting inflammations, cancer could be brought under control. Currently, Nonsteroidal anti-inflammatory drugs, (NSAID) has been associated with a decrease in cancer risk [6, 7]. Aspirin, Diclofenac and Celecoxib are among the most-studied NSAID for cancer therapy. However, the long-term use of NSAID could result in potentially life-threatening conditions [8, 9]. Given the wealth of evidence available from medicinal plant researches, some plants have demonstrated potent activities on cancer and inflammation. Very recently, extracts of Cordia myxa; demonstrated both anti-cancer and anti- inflammatory activities and has been suggested as a potential substitute for NSAID [9].
In this research, the Phytochemical screening and in vivo evaluation of the anti-inflammatory activities of Rutidea parviflora; a Nigerian plant with significant anti-cancer activities {IC50 values of <10 µg/ml in OVCAR-4, OVCAR-8, A2780, and cisplatin resistant A2780 (A2780cis) ovarian Cancer cell lines} is presented.
Materials and Methods
I Plant Materials and Reagents
Rutidea parviflora (root bark) was sourced from a bio reserve in Nigeria. The plants were authenticated by a botanist, Mr. Alfred O. Ozioko, a botanist at the International Centre for Ethnomedicine and Drug Development (INTERCEDD) with expert advice offered by Prof. J.F Bamidele of the Department of Plant Biology and Biotechnology, University of Benin, Nigeria. The voucher specimen number of the plant given as; Rutidea parviflora INTERCEDD/1588. The root bark was dried, dusted, cleaned and cut into pieces before it was milled to coarse powder. Methanol, dichloromethane, chloroform, diethyl ether, acetic anhydride, glacial acetic acid, sodium picrate, 2% 3,5-dinitrobenzoic acid, picric acid, iodine solution, dimethyl sulfoxide, (JHD company, Guangdong. Guanghua Sci-Tech. Co. Ltd. China), hydrochloric acid, Million's reagent, Benedict's solution, Wagner's reagent, sodium hydroxide, ferric chloride solution, saturated lead acetate solution, Dragendorff’s reagent, Kedde reagent, ammonia solution, 7.5% potassium hydroxide, Fehling’s solution A and B (Sigma Aldrich Chemicals, St Louis, USA), distilled water, deionized water (Pharmaceutical Chemistry Lab, University of Port Harcourt).
II Assay Materials
Egg albumin, indomethacin 10 mg/kg (oral), normal saline, digital vernier caliper, hand towels, hand gloves.
III Extraction of Plant Materials
The 2.2 Kg of the pulverized root bark were extracted according to the American National Cancer Institute (NCI) method of extraction [10]. The pulverized plant material was macerated in a 1:1 mixture of dichloromethane and methanol for 24 h to obtain the extracts. The procedure was repeated thrice. The ratio of plant material to solvent used was 1:5. The residue was further macerated in methanol for another 24 h to yield the methanol extract, which was combined with the dichloromethane/methanol extract to yield the total organic extract. Deionized water was added to the residue, to obtain the aqueous extract (3.9 g) after freeze drying. The obtained dry extracts were further dried in a desiccator to remove any trace of solvent. The percentage yields of the crude extracts of the plant were calculated as follows:
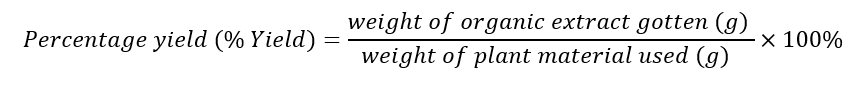
The organic extracts of the plant (24 g, 1.1%) was sequentially partitioned with organic solvents based on increasing order of polarity to yield n-hexane fraction, (7 g) ethyl-acetate fraction, (5.5 g), n-butanol fraction (3.7 g) and aqueous fraction (2.2 g).
IV Phytochemical Screening
Phytochemical tests were carried out on the plants’ extract by the method described by Trease and Evans [11] highlighted below.
i Test for Flavonoids
a. Shinoda Reduction Test
100 mg of the plant extract was dissolved in 5ml of hydrochloric acid, a few pieces of magnesium metal were added to 5 ml of each plant extract solution. The formation of orange i.e. a reddish crimson colour signified the presence of flavonoids.
b. Sodium Hydroxide Test for Flavonoids
A small amount of each of the portions was dissolved in water and filtered; to this, 2 ml of 10% aqueous sodium hydroxide was added to produce a yellow colouration. Upon addition of dilute hydrochloric acid, a change in colour from yellow to colourless indicated the presence of flavonoids.
ii Test for Tannins
Ferric Chloride Test
100 mg of plant extract was boiled with 5ml of distilled water for 5 minutes, cooled and filtered, few drops of 5% ferric chloride reagent was added. The formation of green to blue black precipitate indicated the presence of tannins.
iii Test for Alkaloids
100 mg of the plant extracts were heated for about 2 minutes with 5 ml 2M HCl on the steam bath, the mixture was filtered and to 1 ml of the filtered mixture. Thereafter, 2 drops of the following reagents were added and observed. Dragendorff’s reagent, where a reddish precipitate indicates the presence of alkaloid. Wagner’s reagent, development of yellow precipitate indicates the presence of alkaloid.
iv Test for Saponins
a. Frothing Test
100 mg of the plant extract was mixed with 5 ml of water and shaken vigorously for persistent foam, then warmed and observed persistent foaming on warming indicated the presence of saponins.
b. Emulsion Test
100 mg of plant extract was dissolved in 10 ml of water and mixed with 3 drops of olive oil and shaken vigorously then observed for the formation of emulsion which signifies a positive test for saponins.
v Test for Phlobatannins
Hydrochloride Acid Test 100 mg of plant extract was dissolved in water and filtered. The filtrate was boiled with 1% hydrochloric acid. Deposition of a red coloured precipitate indicates a positive test.
vi Test for sterols/Triterpenoids
a. Liebermann-Buchard Test
100 mg of plant extract is dissolved in chloroform and acetic anhydride is added followed by sulphuric acid. Green colour indicates the presence of steroids and pink colour indicates terpenoids.
b. Salkwoski’s Test
100 mg of plant extract was dissolved in 2ml chloroform, then conc. H2SO4 was carefully added to form a layer. The formation of a reddish brown colour at the interface is indicative of a steroidal nucleus.
vii Test for Cardiac glycosides
a. Keller Killiani Test
100 mg of plant extract was dissolved in about 5ml of water, then glacial acetic acid, one drop of 5% FeCl3 and conc. H2SO4 were added. Reddish brown colour that appears at the junction of the two liquid layers and an upper layer that appears to be bluish-green confirms the presence of glycosides.
b. Kedde Test
About 5ml of dissolved extract was treated with a small amount of Kedde reagent (i.e. equal volumes of a 2% solution of 3, 5 dinitrobenzoic acid in menthol and a 7.5% aqueous solution of KOH). Development of a blue or violet colour that fades out in 1-2 hours indicates the presence of cardiac glycoside.
viii Test for Carbohydrates
a. Fehling’s Test
100 mg of the plant extract was mixed with few drops of Fehling’s solution A + B and boiled for a few minutes. A deep blue to green; yellow to red indicates a positive reduction test.
b. Molisch’s Test
100 mg of plant extract was mixed with 5 ml of distilled water. The mixture was boiled for 15 minutes and filtered. 1 ml of Molisch reagent was added to 1ml of the filtrate and shaken; 1 ml of concentrated H2SO4 was added and observed. The presence of reddish ring indicates the presence of carbohydrate.
ix Test for Proteins
About 100 mg of the sample was extracted with distilled water by boiling then filtering.
a. Million's Test
To a little portion of the filtrate in a test tube, 2 drops of Million's reagent was added. Formation of white Precipitate which indicates the presence of protein was watched out for.
b. Picric Acid Test
To a little portion of the filtrate were added drops of Picric acid solution. A yellow Precipitate indicates the presence of protein.
V Experimental Design
This study was designed in line with the ethically approved experimental protocols adopted by the department of Experimental Pharmacology and Toxicology, of the Faculty of Pharmaceutical Sciences, University of Port Harcourt. Adult mixed-gender wistar rats weighing between 170-200g, bred by the animal house unit of the department of Experimental Pharmacology and Toxicology were used for the study. The rats were housed in spacious cages, to allow for free movement at room temperature, sufficient humidity and 12 hourly cycles of light and darkness. The animals had access to standard laboratory animal feed and water. The animals were divided into 12 test groups and 2 control groups with 6 rats per group. Group A received a dose of 200 mg/kg P.O of the plant extracts and Group B received a dose of 400 mg/kg P.O of the plant extracts. Group C and D received indomethacin (10 mg/kg P.O) and normal saline (1 ml/rat P.O) respectively.
i Egg Albumin Induced Rat Paw Edema
The initial paw thickness of each rat was measured using a digital vernier caliper and recorded as To. Edema was induced in the paws of the animals by administering 0.1ml egg albumin sub-plantarly. After 30 minutes of inducing edema, treatments were given to the rats orally according to their body weights after which their paw thickness were determined at time intervals (T) of 1 hour, 2 hours, 3 hours and 4 hours using a digital vernier caliper.
The percentage inhibition was calculated using the formula,
$$ \frac{(Tt-T0)Control-(Tt-T0)treated\ }{(Tt-\ T0\ )\ Control}\times100 $$
VI Statistical Analysis
The analytical tool used was SPSS version 16 for calculation of the mean values ± standard error of mean (SEM). The one-way ANOVA was used to determine statistical difference between means. A p<0.05 was considered statistically significant.
Results
I Results from the Phytochemical Screening
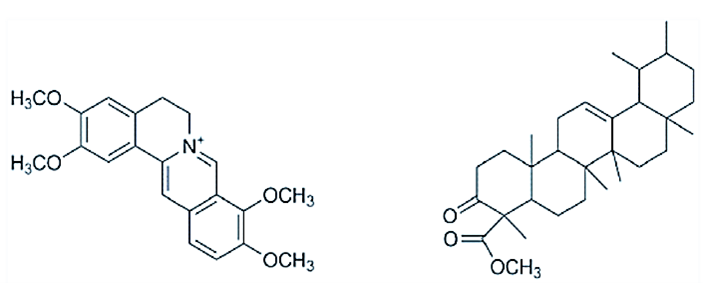
The results of the phytochemical screening are contained in (Table 1). The results presented in (Table 1) showed that most of the phytoconstituents are in the organic extract. Significantly, alkaloids, flavonoids, tannins, terpenoids, and cardiac glycosides are more abundant in the organic extract. Alkaloids such as isoquinoline, indole and diterpene are known to have good anti-inflammatory activity [12]. Previously, an alkaloid; Palmatine and a triterpenoid; urs-12- ene-24-oic acid, 3-oxo, methyl ester were isolated by my research group (See Figure 1 below). Studies have shown that Palmatine possess very good anti-cancer activities in vitro [13].
Figure 1: The chemical structure of palmatine A) and urs-12-ene-24-oic acid, 3-oxo, methyl ester B) isolated from R. parviflora.
Table 1: Results from the phytochemical screening.
|
Test |
R. parviflora
Extracts |
|
|
|
Aqueous |
Organic |
|
Flavonoids |
+ |
++ |
|
Tanins |
+ |
++ |
|
Alkaloids |
Trace |
+++ |
|
Saponins |
++ |
+ |
|
Phlobatannins |
- |
- |
|
Sterols/Terpenoids |
Trace |
+ |
|
Cardiac Glycosides |
+ |
++ |
|
Carbohydrates |
+ |
+ |
|
Proteins |
+ |
+ |
Key: -: Absent; +: Slightly present; ++: Moderately present; +++: Heavily present.
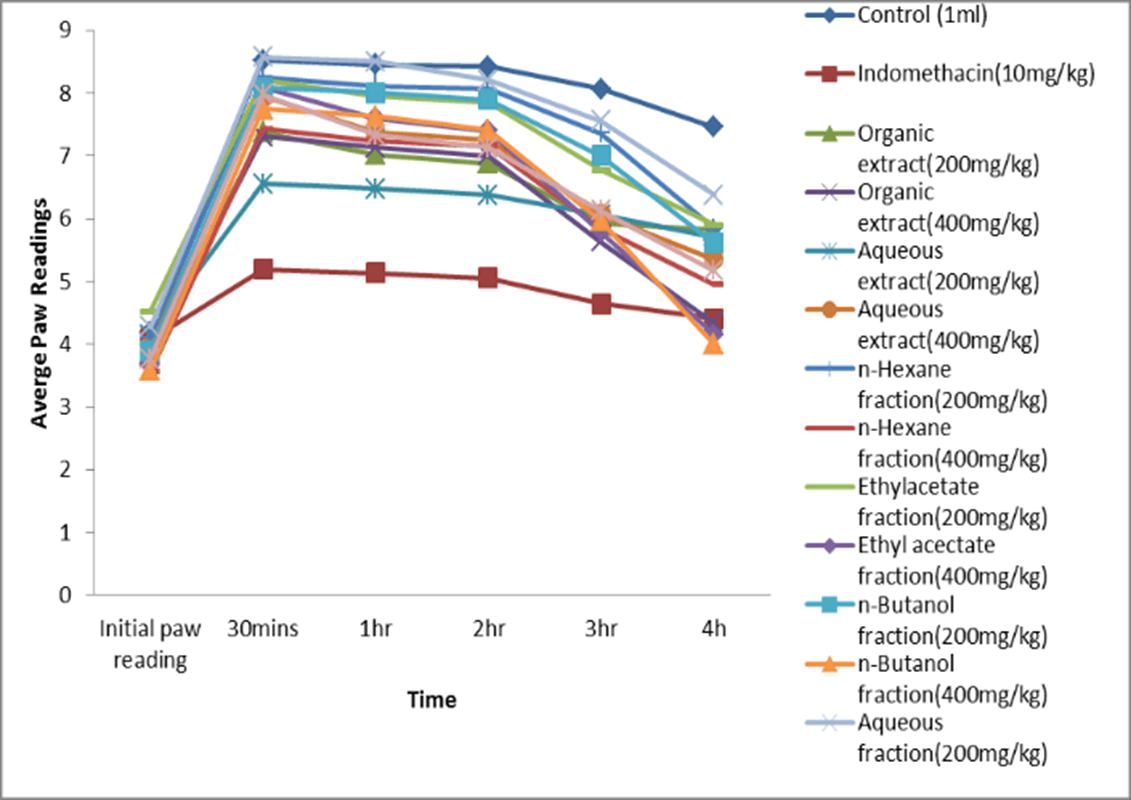
Figure 2: Plot showing the mean paw thickness at time intervals.
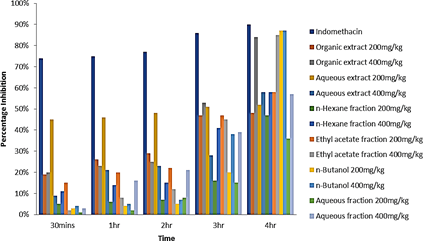
Figure 3: Percentage inhibition of edema of different treatments at time intervals.
II Result for Anti-Inflammatory Assay
Table 2 shows the mean thickness of egg albumin-induced paw edema. At time intervals, each treatment group showed a decrease in mean thickness of the induced paw edema at different rates due to their individual inhibitory effect. From the results, the organic extract (200 mg/kg and 400 mg/kg) had significant anti- inflammatory activity against the negative control from the first hour, (p<0.05), also the Ethyl acetate and n-butanol fractions (200 mg/kg and 400 mg/kg), had significant anti-inflammatory activity from the first hour (1hr) compared to the negative control. However, at the fourth hour when compared to indomethacin (positive control), p>0.05 was obtained, indicating that there was no significant difference between 400 mg/kg organic extract, 400 mg/kg ethyl acetate and 400 mg/kg n-butanol fractions treated groups and indomethacin treated group.
Figure 2 is a graphical representation of the anti-inflammatory activity of the extracts, fractions and the controls. From the experiment it can be deduced that more of the anti-inflammatory activity was found in the ethyl acetate and n-butanol fraction, indicating the active substituents are concentrated more at the polar region of the organic extract. The percentage inhibition of Edema of the different treatments at the respective time Intervals were calculated to obtain the significant values. The data is shown in (Figure 3). The percentage inhibition of indomethacin (10 mg/kg) showed significant inhibition from the 1st, 2nd, 3rd and 4th hour as 75%, 77%, 86% and 90% respectively. The percentage inhibition for the organic extract (400 mg/kg) was significant at the 4th hour as 84%. The percentage inhibition for the ethyl acetate fraction (400 mg/kg) was also significant at the 4th hour (85%). While that of the n-Butanol fraction (200 mg/kg and 400 mg/kg) had a significant inhibition at the 4th hour as 85% and 87% respectively.
Table 2: Mean paw thickness (mm) ± standard error of mean.
|
Treatment |
Initial paw reading |
30mins |
1hr |
2hr |
3hr |
4h |
|
Control (1ml) |
4.18±0.08 |
8.52 ±0.36 |
8.45 0.37 |
8.42 ±0.37 |
8.06 ±0.32 |
7.46 ±0.20 |
|
Indomethacin(10mg/kg) |
4.08±0.15 |
5.19 ±0.07 |
5.13 ±0.06 |
5.5±0.05 |
4.64 ±0.02 |
4.41 ±0.03 |
|
Organic extract(200mg/kg) |
3.86±0.47 |
7.38 ±0.19 |
7.02 0.05* |
6.88 ±0.05* |
5.93 ±0.01* |
5.82 ±0.01* |
|
Organic extract(400mg/kg) |
3.82±0.13 |
7.31 ±0.22 |
7.13 ±0.16* |
7.00 ±0.16* |
5.63 ±0.23* |
4.34 0.30*# |
|
Aqueous extract(200mg/kg) |
4.15±0.04 |
6.55 ±0.21 |
6.47 ±0.24 |
6.37 ±0.19* |
6.06 ±0.04* |
5.71 ±0.05* |
|
Aqueous extract(400mg/kg) |
3.98±0.07 |
7.94 ±0.03 |
7.37 ±0.09* |
7.26 ±0.10* |
6.07 ±0.12* |
5.35 ±0.19* |
|
n-hexane fraction(200mg/kg) |
4.11±0.05 |
8.25 ± 0.14 |
8.11 ±0.14 |
8.06 ±0.13* |
7.36 ±0.32* |
5.84 ±0.33* |
|
n-hexane fraction(400mg/kg) |
3.56±0.21 |
7.43 ±0.31 |
7.27 ±0.36 |
7.16 ±0.35* |
5.85 ±0.14* |
4.95 ±0.03* |
|
Ethylacetate fraction(200mg/kg) |
4.53±0.24 |
8.20 ±0.24 |
7.95 ±0.12* |
7.85 ±0.12* |
6.77 ±0.11* |
5.90 ±0.06* |
|
Ethylacectate fraction(400mg/kg) |
3.67±0.15 |
8.08 ±0.12 |
7.60 ±0.35* |
7.40 ±0.42* |
5.79 ±0.39* |
4.15 0.28*# |
|
n-butanol fraction(200mg/kg) |
3.88±0.09 |
8.08 ±0.21 |
8.00 ±0.23* |
7.89 ±0.25* |
7.00 ±0.04* |
5.61 ±0.14* |
|
n-butanol fraction(400mg/kg) |
3.58±0.29 |
7.75 ±0.36 |
7.63 ±0.31* |
7.41 ±0.25* |
5.97 ±0.14* |
4.00 0.19*# |
|
Aqueous fraction(200mg/kg) |
4.28±0.06 |
8.57 ±0.22 |
8.50 ±0.20 |
8.20 ±0.18* |
7.56 ±0.28* |
6.37 ±0.27* |
|
Aqueous fraction(400mg/kg) |
3.77±0.09 |
7.97 ±0.04 |
7.35 ±0.25* |
7.13 ±0.24* |
6.13 ±0.23* |
5.17 ±0.15* |
Key: Mean±S.E.M. (*) means the p-value is less than 0.05 Significance against control at a given time *p<0.05 Significance against indomethacin at a given time # p>0.05.
Discussion
The observed anti-inflammatory effect is could be attributed to the presence of palmatine, urs-12-ene-24-oic acid, 3-oxo, methyl ester, flavonoids, saponins and other phytochemical constituents in the plant. Flavonoids possess significant activity against both phases of inflammation which are the proliferative and exudative phases. There are a number of reports of saponins with anti-inflammatory properties such as Frutice saponin B [12]. It has been suggested that the numerous biological activities of saponins are linked to their amphiphilic nature which help in the accomplishment of these activities thereby eliciting a cellular response [14].
Urs-12-ene-24-oic acid, 3-oxo, methyl ester, a triterpene, I isolated from the ethyl acetate fraction of R. parviflora, has been reported in the GC-MS analysis of Nothapodytes nimmoniana (Graham) Mabb., Previously, known as Nothopodytes foetida Sleymer and Mappia foetida Miers (Icacinaceae), from which camptothecin, an isoquinoline alkaloid was isolated and currently in clinical use for the treatment of colorectal and ovarian cancer [15]. This compound has also been identified from the GC-MS analysis of the ethanolic extract of the whole plant; Canscora perfoliata used in the treatment of poisonous bites [16]. This compound is an ursolic acid derivative. Ursolic acid from previous studies has been shown to exert cytotoxic activities on a number of cancer cell lines, which includes A2780 and SK-OV3 ovarian cancer cell lines, HT29 colon cancer cell line and PC-3 prostate cancer cell line [17, 18]. Recently the anti-cancer activities of some ursolic acid derivatives have been reported [19, 20].
Previous researchers have documented the anti- asthma, anti-arthritic, anti-inflammatory, anti- microbial and diuretic activities of Urs-12-ene- 24-oic acid, 3-oxo-methyl ester [21]. Palmatine, an isoquinoline alkaloid which belongs to the class of protoberberines, was isolated from the n-butanol fraction of R. parviflora. The compound is yellow in colour and has been isolated from species of plant families such as representatives of Berberidaceae, Ranunculaceae, Menispermaceae and Papaveraceae, which feature prominently in Asian indigenous medicine in the treatment of diseases such as dysentery, hypertension, inflammation, jaundice and liver diseases [22]. The agent demonstrated anti‐inflammatory effects in a dose‐dependent manner in a study carried out in mice treated with three doses of 20, 100, and 200 mg/kg of palmatine [23]. Recently, phytochemical studies of Mahonia bealei, a renowned Chinese medicinal plant reputed for the treatment of gastro-intestinal inflammatory diseases, revealed that palmatine is one of the active compounds contained in the plant. The agent was reported to have inhibited gut tumorigenesis, by targeting inflammatory cytokines [24]. Since this compound has demonstrated both anti-cancer and anti- inflammatory activities in several studies; palmatine, is most likely the key compound responsible for the observed anti-inflammatory activity of R. parviflora.
Conclusion
In conclusion, the result presented in this study suggests that the extract and fractions that were obtained from R. parviflora root-bark possess significant anti-inflammatory activity. From the results, the anti-inflammatory activity of R. parviflora has a slow onset of action. However, the anti-inflammatory effect of n-butanol fraction of R. parviflora at a dose of 400 mg/kg was 87% compared to that of Indomethacin (10 mg/kg); which was 90%, at the 4th hour. The precise mechanisms that are involved in the production of the anti-inflammatory response by R. parviflora fractions are not completely understood but may be due to the presence of palmatine and urs-12-ene-24-oic acid, 3-oxo, methyl ester in the root-bark of R. parviflora.
Conflicts of Interest
None.
Acknowledgements
Our sincere appreciation goes to Mr. Stanley Onyegam for his kind assistance in the management of the animals used in this study.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 16, Sep 2020Accepted: Mon 28, Sep 2020
Published: Fri 30, Oct 2020
Copyright
© 2023 Johnson-Ajinwo Okiemute Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.10.02
Author Info
Johnson-Ajinwo Okiemute Rosa Udofia Cynthia Emmanuel Nwanosike Ahamefula Okeosisi
Corresponding Author
Johnson-Ajinwo Okiemute RosaDepartment of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Nigeria
Figures & Tables
Table 1: Results from the phytochemical screening.
|
Test |
R. parviflora
Extracts |
|
|
|
Aqueous |
Organic |
|
Flavonoids |
+ |
++ |
|
Tanins |
+ |
++ |
|
Alkaloids |
Trace |
+++ |
|
Saponins |
++ |
+ |
|
Phlobatannins |
- |
- |
|
Sterols/Terpenoids |
Trace |
+ |
|
Cardiac Glycosides |
+ |
++ |
|
Carbohydrates |
+ |
+ |
|
Proteins |
+ |
+ |
Key: -: Absent; +: Slightly present; ++: Moderately present; +++: Heavily present.
Table 2: Mean paw thickness (mm) ± standard error of mean.
|
Treatment |
Initial paw reading |
30mins |
1hr |
2hr |
3hr |
4h |
|
Control (1ml) |
4.18±0.08 |
8.52 ±0.36 |
8.45 0.37 |
8.42 ±0.37 |
8.06 ±0.32 |
7.46 ±0.20 |
|
Indomethacin(10mg/kg) |
4.08±0.15 |
5.19 ±0.07 |
5.13 ±0.06 |
5.5±0.05 |
4.64 ±0.02 |
4.41 ±0.03 |
|
Organic extract(200mg/kg) |
3.86±0.47 |
7.38 ±0.19 |
7.02 0.05* |
6.88 ±0.05* |
5.93 ±0.01* |
5.82 ±0.01* |
|
Organic extract(400mg/kg) |
3.82±0.13 |
7.31 ±0.22 |
7.13 ±0.16* |
7.00 ±0.16* |
5.63 ±0.23* |
4.34 0.30*# |
|
Aqueous extract(200mg/kg) |
4.15±0.04 |
6.55 ±0.21 |
6.47 ±0.24 |
6.37 ±0.19* |
6.06 ±0.04* |
5.71 ±0.05* |
|
Aqueous extract(400mg/kg) |
3.98±0.07 |
7.94 ±0.03 |
7.37 ±0.09* |
7.26 ±0.10* |
6.07 ±0.12* |
5.35 ±0.19* |
|
n-hexane fraction(200mg/kg) |
4.11±0.05 |
8.25 ± 0.14 |
8.11 ±0.14 |
8.06 ±0.13* |
7.36 ±0.32* |
5.84 ±0.33* |
|
n-hexane fraction(400mg/kg) |
3.56±0.21 |
7.43 ±0.31 |
7.27 ±0.36 |
7.16 ±0.35* |
5.85 ±0.14* |
4.95 ±0.03* |
|
Ethylacetate fraction(200mg/kg) |
4.53±0.24 |
8.20 ±0.24 |
7.95 ±0.12* |
7.85 ±0.12* |
6.77 ±0.11* |
5.90 ±0.06* |
|
Ethylacectate fraction(400mg/kg) |
3.67±0.15 |
8.08 ±0.12 |
7.60 ±0.35* |
7.40 ±0.42* |
5.79 ±0.39* |
4.15 0.28*# |
|
n-butanol fraction(200mg/kg) |
3.88±0.09 |
8.08 ±0.21 |
8.00 ±0.23* |
7.89 ±0.25* |
7.00 ±0.04* |
5.61 ±0.14* |
|
n-butanol fraction(400mg/kg) |
3.58±0.29 |
7.75 ±0.36 |
7.63 ±0.31* |
7.41 ±0.25* |
5.97 ±0.14* |
4.00 0.19*# |
|
Aqueous fraction(200mg/kg) |
4.28±0.06 |
8.57 ±0.22 |
8.50 ±0.20 |
8.20 ±0.18* |
7.56 ±0.28* |
6.37 ±0.27* |
|
Aqueous fraction(400mg/kg) |
3.77±0.09 |
7.97 ±0.04 |
7.35 ±0.25* |
7.13 ±0.24* |
6.13 ±0.23* |
5.17 ±0.15* |
Key: Mean±S.E.M. (*) means the p-value is less than 0.05 Significance against control at a given time *p<0.05 Significance against indomethacin at a given time # p>0.05.
References
- Virchov R (1863) Cellular pathology as based upon physiological and pathological histology. J B Lippincott Philadelphia.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860-867. [Crossref]
- Askeland EJ, Newton MR, O'Donnell MA, Luo Y (2012) Bladder cancer immunotherapy: BCG and beyond. Adv Urol 2012: 181987. [Crossref]
- Korniluk A, Koper O, Kemona H, Dymicka Piekarska V (2017) From inflammation to cancer. Ir J Med Sci 186: 57-62. [Crossref]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436-444. [Crossref]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP et al. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31-41. [Crossref]
- Todoric J, Antonucci L, Karin M (2016) Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev Res 9: 895-905. [Crossref]
- McGettigan P, Henry D (2011) Cardiovascular risk with non- steroidal anti-inflammatory drugs: systematic review of population- based controlled observational studies. PLoS Med 8: e1001098. [Crossref]
- Ranjbar MM, Assadolahi V, Yazdani M, Nikaein D, Rashidieh B (2016) Virtual dual inhibition of COX-2/5-LOX enzymes based on binding properties of alpha-amyrins, the anti-inflammatory compound as a promising anticancer drug. Excli J 15: 238-245. [Crossref]
- Thomas G (2010) High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active Molecules. Molecules 15: 4526-4563. [Crossref]
- Trease and Evans Pharmacognosy (1989) 15th Ed, India: Elseiver 191- 393.
- Sowjanya R, Shankar M, Sireesha B, Naik EA, Yudharaj P et al. (2017) An overview of inflammation and plant having anti- inflammatory activity. Int J Phytopharm Res 7: 25-32.
- Johnson Ajinwo OR, Richardson A, Li W (2019) Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins (Basel) 11: 237. [Crossref]
- Hassan HS, Sule MI, Musa AM, Musa KY, Abubakar MS et al. (2012) Anti-Inflammatory Activity of Crude Saponin Extracts from Five Nigerian Medicinal Plants. Afr J Tradit Complement Altern Med 9: 250-255. [Crossref]
- Kavitha S, Packia LM, Mary JKS, Mohan VR (2014) GC-MS analysis of ethanolic extract of Nothapodytes nimmoniana (Graham) Mabb. Leaves. Malaya J Biosciences 2: 42-49.
- Kumari S, Muthukumarasamy S, Mohan VR (2012) GC-MS determination of bioactive components of Canscora perfoliata Lam. (Gentianaceae). J App Pharm Sci 2: 210.
- Bai K, Yu Z, Chen F, Li F, Li W et al. (2012) Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg Med Chem Lett 22: 2488-2493. [Crossref]
- Song Y, Jeong S, Kwon H, Kim B, Kim S et al. (2012) Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biol Pharm Bull 35: 1022-1028. [Crossref]
- Chen H, Gao Y, Wang A, Zhou X, Zheng Y et al. (2015) Evolution in Medicinal Chemistry of Ursolic Acid Derivatives as Anticancer Agents. Eur J Med Chem 92: 648-655. [Crossref]
- Shao J, Dai Y, Xue J, Wang J, Lin F et al. (2011) In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur J Med Chem 46: 2652. [Crossref]
- Ramalingam V, Rajangam U (2015) Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Ethanolic Extracts of Aerva lanata (L.). Int J Biochem Res Rev 7: 192.
- Dominik T, Wirginia K (2020) Palmatine: A review of pharmacological properties and pharmacokinetics. Phytother Res 34: 33- 50. [Crossref]
- Küpeli E, Koşar M, Yeşilada E, Başer K (2002) A comparative study on the anti‐inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci 72: 645-657. [Crossref]
- Wei Kun M, Hui L, Cui LD, Xin H, Chang RG et al. (2016) Palmatine from Mahonia bealei attenuates gut tumorigenesis in ApcMin/+ mice via inhibition of inflammatory cytokines. Mol Med Rep 14: 491-498. [Crossref]
