Neutrophil Extracellular Traps: As Antimicrobial Peptides
Neutrophil Extracellular Traps: As Antimicrobial Peptides
A B S T R A C T
Neutrophils are an integral part of innate immune response system, abundantly present in blood circulation. They are the primary responders to the injury or intruding pathogens in human body. Neutrophils engulf infectious microorganisms by the process of phagocytosis, which usually initiates the production of reactive oxygen species and adhere the neutrophilic antimicrobial granules with vacuoles containing pathogens. Upon activation, neutrophils also render signals for stimulation and maturation of macrophages and dendritic cells. They release neutrophil extracellular traps for the suppression of infection and inflammation along with other antimicrobial molecules. The antimicrobials that are present in neutrophil extracellular traps not only eradicate microbes but also moderately contribute to the pathogenesis of various diseases such as destruction of tissue observed in periodontitis. Genetic shortcomings in neutrophils with respect to their chemotaxis, migration and phagocytosis become evident as severe forms of periodontitis, thus highlighting their role in innate immunity. Therefore, the present review is undertaken to highlight the importance of production and release of neutrophil extracellular trap in the regulation of immune reaction and its role in periodontal disease. A comprehensive database search was performed to gather all the relevant data related to the action of neutrophil and neutrophil extracellular traps in various inflammatory diseases with special emphasis on periodontitis.
Keywords
Antimicrobial peptides, innate immunity, neutrophil extracellular traps (NETs), NETosis, periodontal disease, toll like receptors
Introduction
Neutrophils are the specialized phagocytes that counter act external stimulus and infectious pathogens. They rapidly approach the site of infection and destroy the invading bacteria and other microbes through phagocytosis and/or degranulation [1]. Although, degranulation is a more rapid technique of killing microbes extracellularly, it is rather unspecific and can cause harm to the normal healthy tissues of the host [2]. A novel defense mechanism by which neutrophils counteract the pathogens is a DNA based web like structures called neutrophil extracellular traps (NETs) [3]. They are largely composed of decondensed chromatin and other peptides with antimicrobial properties [3]. NETs formation can be initiated by a wide range of stimulators which includes pathogens and their products along with several chemical agents (Table 1) [4]. NETs act by immobilizing various microbes like gram positive and gram-negative bacteria, parasites etc. and contribute towards enhancement of antimicrobial action of neutrophils. Neutrophils via formation of NETs play an important role in several infectious and non-infectious diseases, for example gout, ulcerative colitis, chronic obstructive pulmonary disease (COPD), etc. [5-7]. However, neutrophils can also cause severe damage to the normal healthy tissues due to their detrimental capacity, which is observed in various autoimmune disorders [8]. Considering the facts available from the literature review, interactions between NETs and the pathogens, account for some controversial outcomes concerning the specificity of neutrophils. Here, we discuss about some recent insights into the physiology of neutrophils and NETs, and their role in different diseases with special emphasis on periodontitis.
Neutrophils their morphology and role in innate immunity
Neutrophils are the highly motile, short-lived granulocytes, which act as the first line of defense of human body against invading microbes. They are terminally differentiated phagocytes which comprise the major portion (~ 40-60%) of white blood cell population and play an important role in the innate immunity response [9]. Neutrophils along with other granulocytes are derived from the pluripotent stem cells of the bone marrow. They are produced by sequential differentiation of the myeloblast cells and further migrates from bone marrow tissues into the blood circulation [10]. The neutrophils attack the microbes which involves a number of receptors like toll like receptors (TLRs), Fcγ receptors etc. [11]. When the pathogen binds to any of these receptors, it activates the neutrophils to entrap the microorganism within a vacuole called as phagosome. The enzymes present in the neutrophil are then gets released to destroy the microorganism [12].
Table 1: Factors which stimulates the formation of NETs.
|
MICROBIAL FACTORS |
CHEMICAL FACTORS |
|
A. fumigates |
δ-Toxin from S. epidermidis |
|
C. albicans |
Antibodies |
|
C. gattii |
Calcium ions |
|
C. neoformans |
Glucose oxidase |
|
E. bovis |
GM-CSF +C5a |
|
E. faecalis |
GM-CSF + LPS |
|
E. coli |
Hydrogen peroxide |
|
H. influenza |
Interferon-α + C5a |
|
H. pylori |
Interleukin-8 |
|
K. pneumonia |
LPS |
|
L. lactis |
M1 protein |
|
L. amazonensis donovani/major/chagasi |
Nitric oxide |
|
L. monocytogenes |
Phorbol-12-myristate-13-acetate (PMA) |
|
M. haemolytica |
PMA + ionomycin |
|
M. tuberculosis/canetti |
Platelet activating factor |
|
S. marcescens |
TLR-4 |
|
S. flexneri |
TNF-α |
|
S. aureus |
|
|
S. dysgalactiae |
|
|
S. pneumonia |
|
|
Y. enterocolitica |
|
The morphology of neutrophils is characterized by presence of lobulated nucleus and granules. Due to the presence of multiple lobules in their nucleus, these cells are often called “polymorphonuclear”. The granules present in the neutrophils are vesicles filled with specific contents, depending on which these granules are further classified into primary (contains cathepsin G, myeloperoxidase), secondary (contains lactoferrin, matrix metalloproteinase-8) and tertiary group (contains gelatinase, matrix metalloproteinase-9) [10, 13]. Neutrophils usually exist in an inactive state in the healthy human blood. They can get activated by various factors like microbial products and cytokines or chemokines e.g. granulocyte macrophage colony stimulating factor (GM-CSF), interleukin-8 and interferon-γ, and tumor necrosis factor-α (TNF-α) [9].
Upon activation, neutrophils entrap the microbes and turn them into phagosomes, where two events which are required for antimicrobial activity take place. Initially, the preformed nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits adhere at the phagosomal membrane and transfer the electrons for the formation of superoxide anions [14]. Further, these superoxide anions rearrange and forms dioxygen and the hydrogen peroxide molecules. Altogether, dioxygen, superoxide anions, and hydrogen peroxide are called the reactive oxygen species (ROS) [15]. Now, the granules fuse with the phagosome, exposing the pathogen to large concentrations of ROS, along with different lytic enzymes like proteases, phospholipases etc. which are collectively responsible for their antimicrobial action [16].
Neutrophil extracellular traps
NETs are comprised of a DNA back bone decked up with several histone proteins along with granular peptides. They are produced from activated neutrophils by the process named NETosis. Production of NETs accompanies the generation of higher levels of antimicrobial molecules that kill microbes [17, 18]. NETs production is not followed by apoptosis process because the formation of NETs starts within 10-15 minutes after the cellular activation as compared to the apoptosis process [3]. Formation of NETs has been reported in various species like humans, mice, cats, chinchillas, fish, rabbits, chickens, horses and cows [3, 19-31].
I NETosis
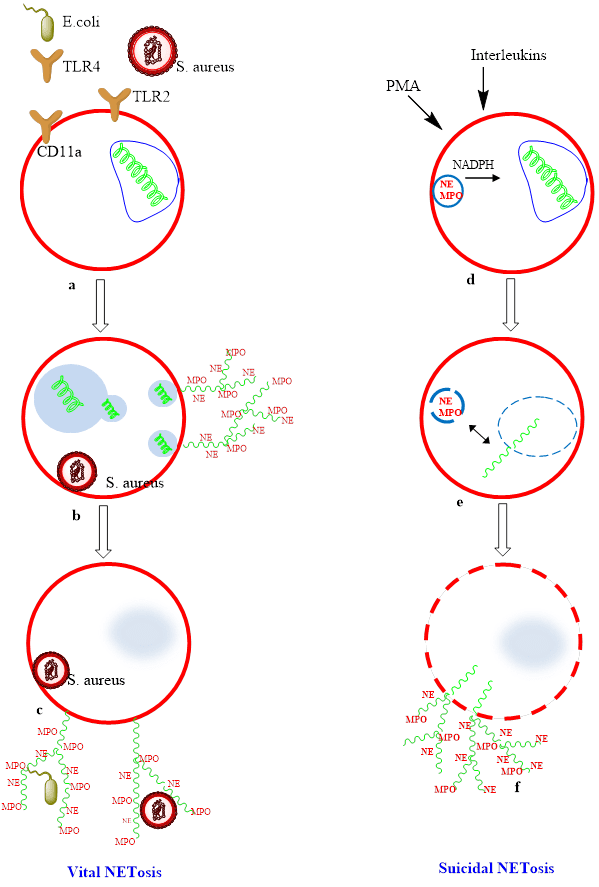
Till date, two mechanisms of NETs production viz. suicidal and vital NETosis have been reported (Figure 1). Suicdial Netosis is the primary route of NETs formation characterized with slow lytic cell death mechanism. It involves the change in characteristic lobulated structure of nucleus which leads to the rupture of nuclear membrane, but the plasma membrane remains intact [32]. The decondensation of chromatin takes place in the cytoplasm where granular proteins get combine with DNA and histones. Afterwards, NETs are released into the extracellular space subsequent to the rupture of plasma membrane. Suicidal NETosis is a slow process and it may take up to 2-4 hours after stimulation. This process was earlier considered to be initiated by ROS and NADPH oxidase [33]. However, the exact mechanism for the involvement of ROS in NETs formation is yet to be understood [34, 35]. It is an important fact that suicidal NETosis is not similar to necrosis and apoptosis processes. Suicidal NETosis does not involve caspase enzyme unlike the apoptosis. Furthermore, the morphological changes during these processes are entirely different [36].
Figure 1: Suicidal NETosis vs Vital NETosis. (A-C) Vital NETosis: Live S aureus induce rapid NET release. For gram-negative bacteria, NETs are induced via Toll-like receptor (TLR) 4 activation of platelets followed by direct neutrophil-platelet interaction via CD11a, whereas both complement receptor 3 and TLR2 are required for gram-positive infection. NETs are released via nuclear budding (D-F) Suicidal NETosis occurs following stimulation by PMA through activation of protein kinase C and the raf–mitogen-activated protein kinase (MEK)–extracellular signal-regulated kinase (ERK) pathway. NADPH assists in the translocation of neutrophil elastase from cytosolic granules into the nucleus where it aids in chromatin breakdown via histone cleavage. MPO is required for chromatin and nuclear envelope breakdown and granular mixing within the NET vacuole. Following 120 minutes of intracellular NET formation, the neutrophil outer membrane ruptures, and the mature NET is extruded.
The second strategy, called vital NETosis. It is a rapid process, undertaken by a small number of neutrophils. This pathway begins with the detachment of nuclear membrane followed by the decondensation of chromatin. Neutrophils apparently release their vesicles which hold the antimicrobial proteins and decondensed chromatin into the extracellular space. This method takes place quickly with 5-60 min as compared to that of suicidal NETosis [3, 37]. It has also been reported that there is another mechanism of NETs formation by the release of mitochondrial DNA [38]. The remaining anuclear neutrophils still maintain their ability of chemotaxis and phagocytosis due to the expression of an apoptotic inhibitor protein, survivine. Mature neutrophils are able to produce survivine after the stimulation from GM-CSF [39-41].
II Morphology of NETs
NETs comprise of stacks of modified forms of nucleosomes approximately 17 nm of diameter. Nucleosome is a subunit of chromatin, composed of DNA wrapped around the histone proteins [42]. NETs contains the globuli (30-50 nm diameter) filled with several cathelicidin peptides along with DNA and histone proteins [43]. When the NETs are treated with DNase enzyme, they are degraded, but when they are treated with proteases enzymes, they retain their structural integrity. This infers that the DNA constituents present in the NETs are of more significance in maintaining the composition of NETs than the protein component [3]. The NETs found in murine are different from that found in human as the globuli found in the murine NETs were closely packed which also covered the entire surface [44].
III Molecular mechanism of NETs production
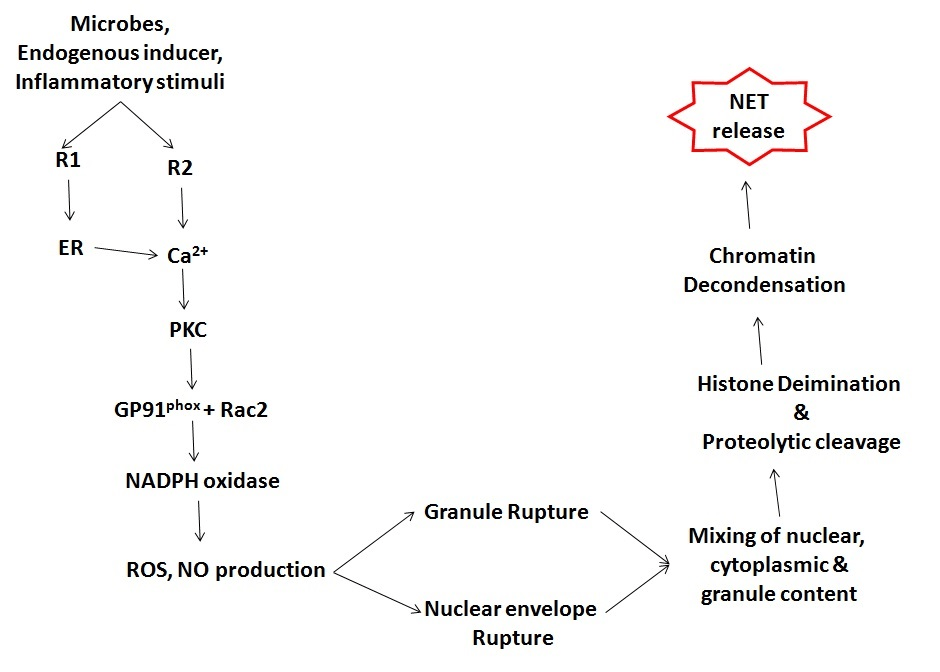
NETs formation is a continuous process which involves various steps: 1. ROS generation via activation of the NADPH-oxidase enzyme; 2. decondensation of chromatin due to the replacement of arginine amino acid with that of citrulline (deimination); 3. transfer of neutrophil elastase and myeloperoxidase from the granules to the nucleus; 4. disruption of cytoplasmic membrane and release of chromatin into the extracellular space (Figure 2) [45].
Involvement of ROS in production of NETs has been observed in the patients suffering from chronic granulomatous disease [46]. Mutation in NADPH oxidase subunits results in the formation of malformed enzyme complex which is unable to synthesize ROS. People with such mutations are more prone to suffer from recurrent lifelong infections [46-48]. The web-like network of the NETs operates by trapping the infectious pathogens and preventing their movement inside the tissues, thus subsequently inactivating the pathogens and eventually removing them out.
Figure 2: NETosis is initiated by binding of neutrophil surface receptors (R) to microbes or microbial breakdown products, inflammatory stimuli, or endogenous inducers. Binding to receptor(s) (exemplified in diagram as R1 and R2) induces endoplasmic reticulum calcium store release and opening of membrane channels that lead to cytoplasmic calcium increases. Elevated calcium stimulates PKC activity, phosphorylation of gp91phox, and assembly of functional NADPH oxidase, leading to ROS and NO production. Morphological changes observed during NETosis include breakdown of nuclear and granule membranes and the mixing of nuclear, granular, and cytoplasmic contents. Proteolytic cleavage of histones may initiate before nuclear breakdown and contribute to chromatin decondensation. A rupture in the plasma membrane allows the release of extracellular chromatin traps.
IV Antimicrobial activity of NETs
Production and the release of NETs take place at a very crucial position, as these traps hinder other important functions of the neutrophils like chemotaxis and phagocytosis. Depending upon the type of stimulus, the neutrophils may or may not survive after the NETs release and may lose their functional capabilities [10]. The most evident in vivo function of NETs in higher organisms is to fight against invading pathogens. NETs are successful against the gram-negative and gram-positive bacterias (E. coli, S. flexneri, S. aureus etc.) and also effective in combating against a wide variety of pathogens like the intracellular parasites such as T. gondii and fungi such as C. albicans [49, 50]. As the NETs are large extracellular structures, it is reasonable that they are efficient in destroying the pathogens which are much larger than the neutrophils itself.
The antimicrobial activity of NETs includes two main stages:
i Trapping and immobilization of microbes to check their dissemination
NETs have been suggested to show the antimicrobial activity due to their ability to physically adhere to the invading pathogens and trap them, which is supported by direct high-resolution imaging evidences of microbial capture, in case of several different pathogens [51]. The preliminary analysis of NETs using electron microscopy and immunofluorescent imaging techniques confirmed their capability to adhere to the microbes like S. aureus, S. typhimurium and S. flexneri after their stimulation [3].
ii Destruction of pathogen by the antimicrobial enzymes present in NETs
There are some controversies about the antimicrobial activity of NETs. Proteases, antimicrobial enzymes, various types of histones and DNA form a part of NETs and contribute toward direct antimicrobial effects. Histones have antibacterial action but under what conditions they were present outside the nucleus is still unclear. Conventionally, neutrophils act by the phagocytosis, but when cytochalasin D is used to inhibit phagocytosis, they still maintained their antibacterial activities [3, 52]. Hence, NETs have their own ability to destroy the microbes.
Neutrophil extracellular traps in infection and autoimmune diseases
NETs prevent the dissemination of infection by localizing the high concentration of antimicrobial agents at infection site [14]. In vitro and in vivo experiments show that both the gram positive and gram-negative pathogens are able to stimulate the formation of NETs either by suicidal or by vital NETosis. Besides phagocytosis and entrapement, the active components of NETs can also neutralize the virulence factors of pathogens [3, 23, 53]. NETs are able to combat the fungal infections effectively. Candida albicans is a eukaryotic pathogen and it causes candidiasis infection in human beings, activates the production of NETs. Both the yeast and hyphal form cells are more prone to destruction by NETs [52]. The antimicrobial protein, calprotectin is involved in neutralizing A. nidulans and prevent its further dispersal. It also showed that calprotectin deficient mice are more prone to aspergillosis infection. Thus this protein appears to be one of the promising factors involved in fungal clearance [42, 46]. C. parapsilosis, a fungal pathogen that causes hematogenously disseminated disease in premature neonates has an escape plan from NETs mediated evasion. C. parapsilosis even after endocytosized by endothelial cells remained alive, multiply intracellularly and eventually burst out of the host endothelial cell [54].
Above facts paved the foundation of antibacterial and antifungual effects of NETs at some extent. NETs also express antiviral factors, such as α-defensin, but the involvement of NETs in antiviral responses remains unclear. NETs capture human immunodeficiency virus (HIV)-1 and promote HIV-1 elimination through myeloperoxidase and α-defensin [55]. Neutrophils in fact perceive HIV-1 by Toll-like receptors (TLRs) TLR7 and TLR8. The role of TLRs is to recognize viral nucleic acids. TLR7 and TLR8 induces the production of reactive oxygen species that trigger NET formation. This process leads to NET-dependent HIV-1 elimination [55]. Various studies depict the imperative role of NETs formation in respiratory infections. Influenza virus is able to initiate the activation of neutrophils and subsequent stimulation of NETs production. Once released, NETs damage the microvascular bed along with the virus mediated destruction of alveolar epithelium and cause acute respiratory distress syndrome. Therefore, therapy involving the inhibition of neutrophil mediated antimicrobial enzymes or destructive molecules will be more appropriate to decrease the severity of influenza pneumonia [56]. A recent study reported the pathogenomic role of NETs in Acute viral bronchiolitis, caused by Respiratory Syncytial Virus (RSV). It is the most common respiratory illness in children along with influenza. RSV fusion protein was able to induce NET release in a concentration-dependent fashion with both neutrophil elastase and myeloperoxidase expressed on DNA fibers [84].
The prospective role of NETs in the elimination of parasites was also considered. NETs form an area that actually contains the microbes but does not kill it. The exact mechanism behind this host threat interaction is yet to be understood. The formation of NETs has been observed after the stimulation with Leishmania species [57]. The protozoan L. mexicana parasite causes chronic non-healing cutaneous lesions in humans and mice with poor parasite control. Parasite sequestration by neutrophils is responsible for disease progression in mice [58]. NETs have been also found in the blood circulation of patients infected with P. falciparum [59]. In addition to the antimicrobial properties, NETs have important role in the pathogenesis of various disease conditions. The interaction of platelets with neutrophils during sepsis triggers the release of NETs. The circulating NETs can injure the endothelium as the histones bind to the endothelial surface where they encourage platelets aggregation and platelet dependent thrombin generation. Concurrently, scaffolds are being formed in the circulation due to the presence of chromatin fibers. These effects led to thrombosis, which causes resistance in the blood circulation. This suggest that NETosis may acutely increase the risks of deep vein thrombosis (DVT) [60]. Thomas GF et al., showed a important link between the cancer and pathogenesis of DVT. This study suggested that tumor factor expressed on tumor microparticles contributes to the increased incidence of cancer associated venous thrombosis in mice in vivo [61]. Contradictory results have been found while studying the role of NETosis in cancer, which needs to be further investigated. It was suggested that NETs may entrap the circulating tumor cells and increase their adhesion to the remote organ sites, thus promoting metastasis. However, the neutrophil elastase enzyme which is the main component of NETs appears to have the capacity to improve tumor cell proliferation. Release of NETs in enhancing the autoimmune response is under investigation [62].
NETs are the potent triggers of inflammation in autoimmune skin diseases, wherein the microbes may use NETs to veil themselves from the adaptive and innate host immunity [63]. Formation of NETs is involved in the pathogenesis of vasculitis, systemic lupus erythematosus, rheumatoid arthritis etc [64, 65, 66]. NETs play a prominent role in the pathogenesis of gout via forming aggregates that densely pack the monosodium urate (MSU) crystals. They further trap and degrade the various pro-inflammatory mediators by intrinsic proteases. Aggregated NETs resemble early stages of the typically large MSU deposits and depicts the pathophysiological structure of gout [6].
Ulcerative colitis (UC) is a major form of inflammatory bowel diseases. UC is caused by an inappropriate immune response to the commensal microorganisms living in the gastrointestinal tract, collectively termed as gut microflora. NETs and the several proteins that play a part in innate immunity are all increased in amount in the morphologically normal colon mucosa from the patients suffering from UC. The increased amount of these antimicrobial compounds points to the stimulation of the innate immune system and its importance in the etiology of UC [7].From the insight of the evidences postulated above, it can be concluded that NETosis mechanism can cause both beneficial and harmful effects. Despite the valuable function of preventing the infection, NETs production participates in the pathogenesis of several diseases, including inflammatory and autoimmune disorders. Thus NETosis can cause unfavorable results, when it occurs in an improper localization or at the wrong time.
Neutrophil extracellular traps in periodontal disease
Periodontitis is a chronic inflammatory condition, which affect the integrity of tooth supporting tissues. Periodontal diseases are one of the major causes of tooth loss in the developed countries [67]. Clinical presentation of periodontitis is marked with destruction of the supporting tissues which mainly occurs due to the abnormal inflammatory response. Although bacteria contribute to the harmful immune response, but tissue damage is predominantly caused by the response of host [68]. As periodontal disease progresses and the gingival crevice deepens to become a periodontal pocket, the microenvironmental conditions become more anaerobic, further aiding the growth of obligate anaerobic bacteria [69]. The severity of periodontitis is characterized by the formation of pockets, clinically estimated by the probing technique. Differential diagnosis of periodontitis is difficult because of widespread presence of microbes in sub-gingival flora [70].
Neutrophils are the first immune response cells to act against gingival infection [71]. Neutrophils express their antibacterial activity. ROS, lipopolysaccharide (LPS) and chemokine, CXCL8 act as possible inducers of NETosis in the gingival crevice [3, 16]. Clinical and histological examination of gingival tissues and crevicular exudate from patients suffering from chronic periodontitis confirms the presence of NETs in abundance at the site of inflammation. NETs trap the crevicular microbes and prevent their adhesion to the gingival tissue. Gingival crevicular samples from chronic periodontitis patients show significantly higher levels of NETs and analysis of the pocket epithelium biopsies also show the presence of NETs [72]. These data are corresponding to those formerly presented by Buchanan et al., in the in vivo assessment of abscess exudates from group A. streptococci infections in mice, and in human mixed bacterial infection [3, 22]. The immune response occurs during periodontitis is rather complex; primarily an innate immune response mounted within the host tissues at the site of infection and ultimately resulting in the activation of acquired immune response and a subsequent increase in antibodies against pathogen found in the gingival crevice or periodontal pocket [68]. At first, this response shows protective function and helps in the phagocytosis of pathogens and prevents their dissemination. The importance of neutrophils for the maintenance of periodontal health can be observed in patients with cyclic neutropenia in which periodontal inflammation and destruction are associated with diminished number of peripheral neutrophils [73]. However, the host inflammatory response can also lead to a destructive ‘hypersensitivity reaction’ which occurs through the release of reactive oxygen species (ROS) and degradative proteolytic agents such as matrix metalloproteinases (MMPs) and serine proteases especially neutrophil elastase [16]. Antimicrobial enzymes like neutrophil elastase present in the granules may also damage contiguous cells and tissues [74]. Also, ROS produced by neutrophils can inactivate protease inhibitors such as α-1 antitrypsin (A1AT), which protects tissues from enzymes of inflammatory cells, especially neutrophil elastase, but the concentration of A1AT may rise in the infection [75, 76]. Peripheral neutrophils from periodontitis patients have been found to be hyper-reactive with regards to ROS generation, and unstimulated neutrophils have higher levels of active elastase and IL-1β than peripheral neutrophils from healthy individuals [77, 78]. Numerous in vitro studies have reported that the hyperreactivity of neutrophils in the patients suffering from chronic periodontitis may cause an excess release of proteolytic enzymes and formation of ROS thus aggravating periodontal tissue destruction [79, 80].
The activity of neutrophils within the periodontal crevice, and its interaction with the pathogens and their role in NETs formation are of huge importance [81]. Dysfunctions in physiological processes like chemotaxis, phagocytosis and superoxide production associated with bactericidal activity of neutrophils is associated with periodontitis [82]. As it involves both the protective and destructive elements, which may be proactively modified by immune resistant pathogens [83].
Conclusion
Neutrophils are the specialized phagocytes which plays an essential role in the defense mechanism against invading pathogens. Upon stimulation, they move towards the site of inflammation and they destroy intruding microbes. Recently, a novel approach of neutrophils action against the invading pathogens has been discovered. NETs are a new innate immune mechanism for microbial inhibition, which are released from the neutrophils. This provides a mechanism by which innate cells can “fight back” or, interfere with bacterial virulence factors extracellularly. NETs act as an effective tool that primarily shields the body from severe infections, but if overproduced, the NETs may provoke some autoimmune disorders, vascular diseases and cancer metastases.
Patients with abnormal genetic makeup with respect to the normal antimicrobial activities of neutrophils will suffer from severe forms of periodontitis. Pathogenesis of periodontitis involves NETosis. The antimicrobial wall is supposed to function in two ways: as a frontier phagocytic molecule and as a robust secretary structure (ROS and bacteriocidal proteins). NETs may proliferate tissue destructive mechanisms in susceptible individuals rather than provide protection on other hand, release of host-derived DNase may play an important role in the digestion and elimination of neutrophil extracellular traps within tissues. Differentiation between the host and microbial DNA is necessary for NETs evasion mechanism and the potential antimicrobial activity. Even though the presence of several facts certifies the important role of NETs in periodontitis but some beads are still missing from the string such as, the structural organization, characteristics and practical significance of the NETs in response to periodontitis. Hence, it is imperative to upgrade the knowledge regarding the mechanisms underlying the production and degradation of NETs and its association with periodontitis.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 03, Aug 2019Accepted: Thu 29, Aug 2019
Published: Fri 13, Sep 2019
Copyright
© 2023 Quratul Ann. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ORD.2019.01.02
Author Info
Corresponding Author
Quratul AnnDepartment of Oral Medicine and Diagnostic Science, College of Dentistry, King Saud University, Riyadh, KSA
Figures & Tables
Table 1: Factors which stimulates the formation of NETs.
|
MICROBIAL FACTORS |
CHEMICAL FACTORS |
|
A. fumigates |
δ-Toxin from S. epidermidis |
|
C. albicans |
Antibodies |
|
C. gattii |
Calcium ions |
|
C. neoformans |
Glucose oxidase |
|
E. bovis |
GM-CSF +C5a |
|
E. faecalis |
GM-CSF + LPS |
|
E. coli |
Hydrogen peroxide |
|
H. influenza |
Interferon-α + C5a |
|
H. pylori |
Interleukin-8 |
|
K. pneumonia |
LPS |
|
L. lactis |
M1 protein |
|
L. amazonensis donovani/major/chagasi |
Nitric oxide |
|
L. monocytogenes |
Phorbol-12-myristate-13-acetate (PMA) |
|
M. haemolytica |
PMA + ionomycin |
|
M. tuberculosis/canetti |
Platelet activating factor |
|
S. marcescens |
TLR-4 |
|
S. flexneri |
TNF-α |
|
S. aureus |
|
|
S. dysgalactiae |
|
|
S. pneumonia |
|
|
Y. enterocolitica |
|
References
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30: 459-89. [Crossref]
- Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM (2013) Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J Leukoc Biol 93: 185-198. [Crossref]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532-1535. [Crossref]
- Zawrotniak M, Rapala-Kozik M (2013) Neutrophil extracellular traps (NETs)-formation and implications. Acta Biochim Pol 60: 277-284. [Crossref]
- Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner WD, Steinbacher P et al. (2015) Neutrophil extracellular trap (NET) formation characterises stable and exacerbated copd and correlates with airflow limitation. Respir Res 16: 59. [Crossref]
- Maueroder C, Kienhofer D, Hahn J, Schauer C, Manger B et al. (2015) How neutrophil extracellular traps orchestrate the local immune response in gout. J Mol Med (Berl) 93: 727-734. [Crossref]
- Bennike TB, Carlsen TG, Ellingsen T, Bonderup OK, Glerup H et al. (2015) Neutrophil extracellular traps in ulcerative colitis: A proteome analysis of intestinal biopsies. Inflamm Bowel Dis 21: 2052-2067. [Crossref]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM (2010) Neutrophil kinetics in health and disease. Trends Immunol 31: 318-324. [Crossref]
- Wright HL, Moots RJ, Bucknall RC, Edwards SW (2010) Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49: 1618-1631. [Crossref]
- Cooper PR, Palmer LJ, Chapple IL (2013) Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol 2000 63: 165-197. [Crossref]
- Garcia-Garcia E, Rosales C (2002) Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol 72: 1092-1108. [Crossref]
- Rosales C (2005) Molecular Mechanisms of Phagocytosis, Springer.
- Brinkmann V, Zychlinsky A (2012) Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol 198: 773-783. [Crossref]
- Branzk N, Papayannopoulos V (2013) Molecular mechanisms regulating netosis in infection and disease. Semin Immunopathol 35: 513-30. [Crossref]
- Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 92: 3007-3017. [Crossref]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I et al. (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176: 231-241. [Crossref]
- Steinberg BE, Grinstein S (2007) unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci Stke 2007: Pe11. [Crossref]
- Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H et al. (2014) Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 13: 690-698. [Crossref]
- Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S (2005) Induction of neutrophil extracellular DNA lattices by placental microparticles and il-8 and their presence in preeclampsia. Hum Immunol 66: 1146-1154. [Crossref]
- Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A et al. (2007) The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 204: 793-804. [Crossref]
- Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A et al. (2006) An endonuclease allows Streptococcus Pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16: 401-407. [Crossref]
- Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA et al. (2006) DNAse expression allows the pathogen group a Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol 16: 396-400. [Crossref]
- Wartha F, Beiter K, Albige B, Fernebro J, Zychlinsky A et al. (2007) Capsule and D-Alanylated lipoteichoic acids protect streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 9: 1162-1171. [Crossref]
- Wardini AB, Guimarães-Costa AB, Nascimento MT, Nadaes NR, Danelli MG et al. (2010) Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J Gen Virol 91: 259-264. [Crossref]
- Hong W, Juneau RA, Pang B, Swords WE (2009) Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus Influenzae persistence in the chinchilla model for otitis media. J Innate Immun 1: 215-224. [Crossref]
- Reid SD, Hong W, Dew KE, Winn DR, Pang B et al. (2009) Streptococcus Pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis 199: 786-794. [Crossref]
- Palić D, Ostojić J, Andreasen CB, Roth JA (2007) Fish Cast Nets: Neutrophil extracellular traps are released from fish neutrophils. Dev Comp Immunol 31: 805-816. [Crossref]
- Chuammitri P, Ostojić J, Andreasen CB, Redmond SB, Lamont SJ et al. (2009) Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet Immunol Immunopathol 129: 126-131. [Crossref]
- Alghamdi AS, Foster DN (2005) Seminal DNAse frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod 73: 1174-1181. [Crossref]
- Lippolis JD, Reinhardt TA, Goff JP, Horst RL (2006) Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet Immunol Immunopathol 113: 248-255. [Crossref]
- Behrendt JH, Ruiz A, Zahner H, Taubert A, Hermosilla C (2010) Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria Bovis. Vet Immunol Immunopathol 133: 1-8. [Crossref]
- Yang Y, Jiang G, Zhang P, Fan J (2015) Programmed cell death and its role in inflammation. Mil Med Res 2: 12. [Crossref]
- Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159-175. [Crossref]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11: 519-531. [Crossref]
- Campbell AM, Kashgarian M, Shlomchik MJ (2012) NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med 4: 157ra141. [Crossref]
- Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E et al. (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21: 290-304. [Crossref]
- Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG et al. (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus Aureus. J Immunol 185: 7413-7425. [Crossref]
- Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A et al. (2012) Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol 167: 261-268. [Crossref]
- Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM et al. (2008) Catapult-like release of mitochondrial dna by eosinophils contributes to antibacterial defense. Nat Med 14: 949-953. [Crossref]
- Yipp BG, Petri B, Salina D, Jenne CN, Scott BN et al. (2012) Infection-induced netosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18: 1386-1393. [Crossref]
- Altznauer F, Martinelli S, Yousefi S, Thurig C, Schmid I et al. (2004) Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med 199: 1343-1354. [Crossref]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida Albicans. Plos Pathog 5: e1000639. [Crossref]
- Brinkmann V, Zychlinsky A (2007) Beneficial suicide: Why neutrophils die to make NETs. Nat Rev Microbiol 5: 577-582. [Crossref]
- Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A et al. (2009) Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 1: 181-193. [Crossref]
- Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG et al. (2013) Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. Plos One 8: e54205. [Crossref]
- Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J (2011) Restoration of anti-aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol 127: 1243-1252. [Crossref]
- Chapple IL, Matthews JB (2007) The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 43: 160-232. [Crossref]
- Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU (2009) Viable neutrophils release mitochondrial dna to form neutrophil extracellular traps. Cell Death Differ 16: 1438-1444. [Crossref]
- Neeli I, Dwivedi N, Khan S, Radic M (2009) Regulation of extracellular chromatin release from neutrophils. J Innate Immun 1: 194-201. [Crossref]
- Grinberg N, Elazar S, Rosenshine I, Shpigel NY (2008) Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia Coli. Infect Immun 76: 2802-2807. [Crossref]
- Engelmann B, Massberg S (2013) Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 13: 34-45. [Crossref]
- Urban CF, Reichard U, Brinkmann V, Zychlinsky A (2006) Neutrophil extracellular traps capture and kill Candida Albicans Yeast and Hyphal Forms. Cell Microbiol 8: 668-676. [Crossref]
- Lauth X, von Kockritz-Blickwede M, Mcnamara CW, Myskowski S, Zinkernagel AS et al. (2009) M1 protein allows group a streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun 1: 202-214. [Crossref]
- Glass KA, Longley SJ, Bliss JM, Shaw SK (2015) Protection of candida parapsilosis from neutrophil killing through internalization by human endothelial cells. Virulence 6: 504-514. [Crossref]
- Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M et al. (2012) Neutrophil extracellular traps mediate a host defense response to human immunodeficiency Virus-1. Cell Host Microbe 12: 109-116. [Crossref]
- Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP et al. (2011) Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179: 199-210. [Crossref]
- Guimaraes-Costa AB, Nascimento MT, Froment GS, Soare RP, Morgado FN et al. (2009) Leishmania Amazonensis Promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A 106: 6748-6753. [Crossref]
- Hurrell BP, Schuster S, Grun E, Coutaz M, Williams RA, Held W et al. (2015) rapid sequestration of leishmania mexicana by neutrophils contributes to the development of chronic lesion. Plos Pathog 11: e1004929. [Crossref]
- Baker VS, Imade GE, Molta NB, Tawde P, Pam SD et al. (2008) Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in plasmodium falciparum infected children under six years of age. Malar J 7: 41. [Crossref]
- Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K et al. (2012) Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 10: 136-144. [Crossref]
- Thomas GM, Brill A, Mezouar S, Crescence L, Gallant M et al. (2015) Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost 13: 1310-1319. [Crossref]
- Cools-Lartigue J, Spicer J, Mcdonald B, Gowing S, Chow S et al. (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 1. [Crossref]
- Stephan A, Fabri M (2015) the net, the trap and the pathogen: Neutrophil extracellular traps in cutaneous immunity. Exp Dermatol 24: 161-166. [Crossref]
- Yu Y, Su K (2013) Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol 4. [Crossref]
- Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL et al. (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623-625. [Crossref]
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S et al. (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5: 178ra40. [Crossref]
- Leong XF, Ng CY, Badiah B, Das S (2014) Association between hypertension and periodontitis: Possible mechanisms. Scientificworldjournal 2014: 768237. [Crossref]
- Guthmiller JM, Novak KF (2002) Periodontal diseases. [Crossref]
- Samaranayake LP (2006) Essential Microbiology for Dentistry. Elsevier Health Sciences.
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontology 2000 14: 216-248. [Crossref]
- Page RC, Kornman KS (1997) The pathogenesis of human periodontitis: An Introduction. Periodontol 2000 14: 9-11. [Crossref]
- Vitkov L, Klappacher M, Hannig M, Krautgartner WD (2009) Extracellular neutrophil traps in periodontitis. J Periodontal Res 44: 664-672. [Crossref]
- Scott DA, Krauss J (2012) Neutrophils in periodontal inflammation. Front Oral Biol 15: 56-83. [Crossref]
- Van Dyke TE, Hoop GA (1990) Neutrophil function and oral disease. Crit Rev Oral Biol Med 1: 117-133. [Crossref]
- Stockley RA (1999) Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med 160: S49-S52. [Crossref]
- Stockley RA (2002) Neutrophils and the pathogenesis of COPD. Chest 121: 151S-155S. [Crossref]
- Figueredo CM, Gustafsson A, Asman B, Bergstrom K (2000) Expression of intracellular elastase activity in peripheral neutrophils from patients with adult periodontitis. J Clin Periodontol 27: 572-577. [Crossref]
- Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR (2007) Neutrophil hyper-responsiveness in periodontitis. J Dent Res 86: 718-722. [Crossref]
- Fredriksson M, Gustafsson A, Asman B, Bergstrom K (1998) Hyper-Reactive peripheral neutrophils in adult periodontitis: generation of chemiluminescence and intracellular hydrogen peroxide after in vitro priming and fcgammar-stimulation. J Clin Periodontol 25: 394-398. [Crossref]
- Gustafsson A, Ito H, Asman B, Bergstrom K (2006) Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J Clin Periodontol 33: 126-129. [Crossref]
- Jayaprakash K, Demirel I, Khalaf H, Bengtsson T (2015) The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas Gingivalis. Mol Oral Microbiol 30: 361-375. [Crossref]
- Meng H, Xu L, Li Q, Han J, Zhao Y (2007) Determinants of host susceptibility in aggressive periodontitis. Periodontol 2000 43: 133-159. [Crossref]
- Gaffen SL, Hajishengallis G (2008) A new inflammatory cytokine on the block: Re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and Il-17. J Dent Res 87: 817-828. [Crossref]
- Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP et al. (2015) Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. Plos One 10: e0124082. [Crossref]
