Multifaceted Networking of The Orphan Receptor Estrogen-Related Receptor Β in Breast Cancer Progression
A B S T R A C T
Breast cancer death polls are rising at an alarming rate in females globally. It is a hormone-dependent disease that is majorly regulated by estrogen. Several genetic and environmental factors are the primary attributes of breast cancer growth and development. A higher proportion of breast cancer patients harbor estrogen receptor-positive (ER+ve) status. Estrogen related receptors are orphan nuclear receptors which include ERRα, ERRβ, and ERRϒ that exhibit a sequence similarity with ERα. ERRα and ERRϒ act as activators in cancer. ERRβ expression is downregulated in breast cancer cells and patient samples, compared to healthy breast cells. The decreased expression of ERRβ is primarily mediated by the proteasomal pathway at the protein level. ERRβ restricts cell cycle progression in breast cancer cells, thereby impeding breast cancer proliferation. Neddylation of ERRβ mediates its downregulation which triggers the oncogenic signalling in breast cancer. Our study showed employing MLN4924, an NAE inhibitor to restore the expressivity of ERRβ could provide a successful and cutting-edge therapeutic method. This review article illustrates the regulatory role of ERRβ in the formation and evolution of breast cancer, making it an effective therapeutic candidate.
Keywords
Breast cancer, estrogen-related receptor β (ERRβ), estrogen receptor α (ERα)
Introduction
Breast cancer is the leading cause of morbidity in females globally, with an anticipated 2.3 million new cases, representing 11.7% of all cancer cases. According to epidemiological studies, the worldwide burden of breast cancer is predicted to exceed 2 million by 2030. Between 1965 and 1985, the incidence grew by over 50% in India. According to the GLOBOCAN statistics 2020, 13.5% (178361) cases of breast cancer and 10.6% (90408) of all cancer deaths in India, with a cumulative risk of 2.81 has been reported [1].
The therapeutic regime for breast cancer has significantly evolved in the past few years. However, for the patients with advanced breast cancer who had first-line chemotherapy induced acquired drug resistance, disease recurrence, or metastasis., treatment constraints still prevail [2]. According to the molecular patterns of gene expression, four intrinsic subtypes of breast cancer include luminal A (ER+/HER), luminal B (ER+/HER2- or HER2+), triple-negative/basal type, and HER2 type [3]. About 60-70% of breast cancer is of the luminal subtype, which is distinguished by the presence of the estrogen receptor (ER), and responds more effectively to endocrine therapy, such as tamoxifen [4]. But because there is no treatment for ER-negative breast cancer, it is necessary to find potential therapeutic molecular targets.
Estrogen receptors (ERs) including ERα, ERβ, and ERγ are nuclear receptors that are mainly regulated by a steroid hormone i.e., estrogen. ERα and Erβ are highly expressed in several tissues, including the brain. ERα promotes cell migration, division, and tumor formation in response to estrogen, whereas ERβ has anticancer activity [5]. Nuclear receptors that fall within the family of estrogen-related receptors (ERRs) function as transcriptional regulators and share sequence homology with ERs [6]. Due to non-availability of natural ligands, they are regarded as orphan nuclear receptors. The target genes of ERs and estrogen-related receptors (ERRs) are shared [7]. The three major different types of estrogen-related receptors (ERRs) are ERRα, ERRβ, and ERRγ. Multiple studies suggest that physiologically ERRβ involved in specific functions during early mouse development, maintenance of pluripotent and multipotent populations of the embryo and primordial germ cells, reprogramming, and self-renewal of embryonic stem cells [8]. Moreover, ERRβ mediates the transit from pluripotency to the early stage of differentiation (Mazloom et. al. 2023). Through acting as a transcriptional activator as well as a repressor, ERRα, and ERRγ are predominantly involved in the metabolism of breast cancer. However, ERRβ function as a tumor suppressor in breast cancer by regulating multiple genes involved in cell proliferation [9]. In triple-negative breast cancer (TNBC) ERRβ mRNA expression is significantly lower with poor overall survival than other breast cancer subtypes [10].
This study illustrates the structure-function, interaction, and mechanism of ERRβ with the other transcription factors, which will aid in the development of novel therapeutic strategies for the growth and development of breast cancer.
Structural Organization of ERRβ
Estrogen-related receptor beta (ERR-β), also known as ESRRB or NR3B2 (nuclear receptor subfamily 3, group B, member 2), is a nuclear receptor that in humans encoded by the ESRRB (Estrogen Related Receptor Beta) gene [11]. The ESRRB gene encodes 433 amino acids having molecular mass 48KDa situated on chromosome 14q24.3 [12]. The 3' end of ERRβ is alternatively spliced to create ERRβ short form (ERRβsf), ERRβ2, and ERRβ exon 10-deleted (ERRβ-Δ10) [13]. The molecular function(s) of endogenous ERRβ splice variants in breast cancer and other tumor types are unknown. As ER, share about 68% sequence homology in DNA binding domain (DBD) with ERR, it is assumed that they may share a target gene [14]. Electrophoretic mobility shift assay (EMSA) results confirmed the binding of ERα on both the half ERE sites situated from - 877 to - 872 and - 810 to - 805 in the upstream region of ERRβ promoter [5].
ERRs control the transcriptional activity of target gene promoters by detecting a short sequence known as an ERR-responsive element (ERRE) with the consensus sequence (TNAAGGTCA) in the promoter region. Estrogen-independent regulation of ERRβ modulates a large number of estrogen-dependent genes [15]. The impact of estrogen on ERs is very high in comparison to ERRβ. However, the estrogen hormone enhances the binding of ERRβ on half EREs through ERα. Re CHIP data suggests ERα and ERR β heterodimer binding to the half ERE sites present on the ERRβ promoter. Whereas ER-negative (ER-ve) breast cancer cells (MDA-MB-231) showed no binding of ERα on the ERRβ promoter as the estrogen hormone receptor is absent [5].
Regulation of ERRβ in Cell Cycle Progression
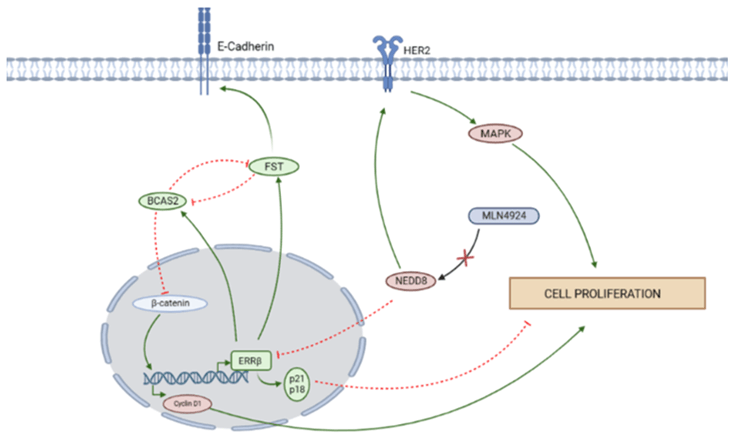
Ectopic regulation of the cell cycle is one of the essential hallmarks of cancer. Uncontrollable cell division is usually driven by multiple mutations that both prevent apoptosis and compromise the withdrawal of the cell cycle. Mutation in molecules involved in DNA damage response, mitogens, spindle assembly, etc helps evade the cell cycle checkpoints which result in the abnormal division of the cell in cancer. ERRβ which is often downregulated in breast cancer is involved in an intricate gene regulation that leads to the suppression of cell proliferation. Sengupta et al. reported that ERRβ inhibits the cell cycle progression at G1 to S transition through regulation of BCAS2 and FST [16]. FST acts tumor suppressor in breast cancer, whereas BCAS2 acts as an oncogene [17]. However, in ERRβ overexpressed cells, BCAS2 inhibits cyclin D1, henceforth halting the cell cycle at G1-S transition and the detrimental effect is neutralized by FST (Figure 1). One of the three alternatively spliced versions of ERRβ have shown a role for this receptor in growth inhibition and cell cycle arrest at the G1 checkpoint in prostate cancer. Heckler reported that splice variants of ERRβ have different functions in cell cycle regulation [18]. ERRβsf promotes cell death and cell cycle arrest at G1, on the contrary ERRβ2 has quite the opposite function. Besides breast cancer, ERRβ is reported to play many roles in other types of malignancies (Table 1).
Table 1: Role of ERRβ across different cancer.
|
Sl
no. |
Cancer |
Role |
Function |
Ref |
|
1. |
Glioblastoma |
Tumor Suppressor gene |
The
study delineates that the long isoform of ERR-β downregulates the
Glioblastoma cell migration due to the interaction with the actin nucleation
factor cortactin. |
[19] |
|
2. |
Prostate
cancer |
Tumor Suppressor gene |
ERRβ
plays a pivotal role in downregulating the expression pattern in ERR-β
transduced and non-transduced cells. The study also reveals that ERR β /γ
agonist DY131 plays a potential role in ERR-β mediated growth inhibition. |
[9] |
|
3. |
Uterine
cancer |
Yet
to be known |
Studies Corroborate the functional
characterization of exogenous ERRβsf and ERRβ2 in the ERα-positive Ishikawa
endometrial cancer cell line. Study reveals that Exogenous ERR β2 enhances ER
α activity upon estrogen induction at an ERE-luciferase reporter, while
ERRβsf abrogated ERα activity. |
[20] |
|
4. |
Ovarian
cancer |
Oncogene |
Studies
reveal the high protein expression of the Orphan receptor ERRβ in the various
subtypes of serous ovarian malignancies which indicates a significantly
shorter overall patient survival. |
[21] |
|
5 |
Breast
Cancer |
Tumor Suppressor gene |
Studies
report the overexpression of ERRβ in patient samples compared to normal
samples. Expression
of ERRβ is Erα dependent and ERRβ is a direct potential target of Erα.
There
is an evident decrease in the expression of ERRB in breast cancer, which is
mediated by the proteasomal pathway via Cullin1. |
[22,
23] |
Figure 1: Diagrammatic representation showing ERRβ regulation in various cell signalling pathways. Created with (Link).
Post Translational Modification of ERRβ in Breast Cancer
Newly translated polypeptides often undergo post-translational modification (PTM) where a variety of small chemical groups i.e., acetylation, methylation, phosphorylation, glycosylation, neddylation, etc are attached covalently to the amino acids. Many of these PTMs have been reported to associate with different aspects of cancer progression. As PTMs are responsible for the stability, folding, and function as well as the interaction of the protein with other proteins, ectopic regulation could lead to detrimental consequences i.e., cancer.
Neddylation is one of the PTMs where NEDD8 is covalently attached to the carboxy terminal of glycine and lysine and is involved in many diseases including breast cancer. It is seen that Neddylation stabilizes HER2 and promotes the progression of breast cancer [23]. Our study illustrates the downregulation of ERRβ by Neddylation which correlates with the poor prognosis in breast cancer [24]. In vivo study using the chick chorioallantoic membrane (CAM) xenograft model confirms that MLN4924, an inhibitor of Neddylation impedes tumor growth. In vitro experiments further conclude that MLN4924 inhibits the proteasomal degradation of ERRβ followed by accumulation of p21 and p27 which hinders the cell cycle progression. ERRβ binds to the promoter region of E- cadherin, and also the coactivator p300 gets recruited to the e-cadherin promoter binding site thereby enhancing its transcription activity. These will eventually attenuate the migratory ability of breast cancer cells.
Conclusion
Breast cancer accounts for 14.3% of all cancer deaths worldwide and is the main cause of cancer fatalities in developing nations. The predominant subtype of breast cancer is ER-positive tumors. ERα becomes activated and forms a heterodimer by interacting with ERRβ in the presence of estrogen. The heterodimer moves into the nucleus, binds to the promoter region of ERRβ, and increases its promoter activity. ERRβ stimulates the expression of cell cycle markers such as p21cip and p18. These markers are the cyclin-dependent kinase inhibitors that stop the cell cycle. ERRβ also acts as a transcriptional up regulator of FST and BCAS2 which helps in arresting cell cycle and apoptosis respectively.
Estrogen-related receptors (ERRs) have sequence homology with ERs and act as transcriptional regulators. The molecular mechanism of orphan nuclear receptor ERRβ in breast cancer is poorly understood. It has been also well-recognized that ERRβ plays an important role in cellular metabolism and its mutation leads to deafness in physiological conditions. In breast cancer patients, it has been observed that estrogen-related receptor beta (ERRβ) expression is decreased, and its overexpression is associated with a better prognosis and longer survival. The downregulation of ERRβ is mediated through the ubiquitin-proteasome pathway. MLN4924, a selective small molecule inhibitor of neddylation, helps in increasing the ERRβ expression and results in the reduction of cell proliferation and migration in breast cancer cells by promoting the expression of significant anti-proliferative and anti-migratory genes such as p21 and e-cadherin. Furthermore, studies show that the ERRβ engages the transcription co-activator p300 to its targeted gene promoters i.e., e cadherin to upregulate their expression. There are a few studies that address the role of ERRβ as an oncogene in cancer. Significant evidence is currently emerging to support these claims and to give light on the mechanisms involved in different pathways. This study uncovers the potential regulation of modulating breast tumorigenesis, that can be used as a novel and effective strategy for breast cancer treatment.
Article Info
Article Type
Review ArticlePublication history
Received: Fri 19, May 2023Accepted: Tue 13, Jun 2023
Published: Mon 26, Jun 2023
Copyright
© 2023 Sandip K. Mishra. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2023.02.01
Author Info
Monalisa Parija Rakesh Padhan G. Kumari Pritish Samal Sandip K. Mishra
Corresponding Author
Sandip K. MishraCancer Biology Lab, Gene Function & Regulation Group, Institute of Life Sciences, Nalco Nagar Road, Nalco square, Chandrasekharpur, Bhubaneswar, Odisha, India
Figures & Tables
Table 1: Role of ERRβ across different cancer.
|
Sl
no. |
Cancer |
Role |
Function |
Ref |
|
1. |
Glioblastoma |
Tumor Suppressor gene |
The
study delineates that the long isoform of ERR-β downregulates the
Glioblastoma cell migration due to the interaction with the actin nucleation
factor cortactin. |
[19] |
|
2. |
Prostate
cancer |
Tumor Suppressor gene |
ERRβ
plays a pivotal role in downregulating the expression pattern in ERR-β
transduced and non-transduced cells. The study also reveals that ERR β /γ
agonist DY131 plays a potential role in ERR-β mediated growth inhibition. |
[9] |
|
3. |
Uterine
cancer |
Yet
to be known |
Studies Corroborate the functional
characterization of exogenous ERRβsf and ERRβ2 in the ERα-positive Ishikawa
endometrial cancer cell line. Study reveals that Exogenous ERR β2 enhances ER
α activity upon estrogen induction at an ERE-luciferase reporter, while
ERRβsf abrogated ERα activity. |
[20] |
|
4. |
Ovarian
cancer |
Oncogene |
Studies
reveal the high protein expression of the Orphan receptor ERRβ in the various
subtypes of serous ovarian malignancies which indicates a significantly
shorter overall patient survival. |
[21] |
|
5 |
Breast
Cancer |
Tumor Suppressor gene |
Studies
report the overexpression of ERRβ in patient samples compared to normal
samples. Expression
of ERRβ is Erα dependent and ERRβ is a direct potential target of Erα.
There
is an evident decrease in the expression of ERRB in breast cancer, which is
mediated by the proteasomal pathway via Cullin1. |
[22,
23] |
References
1. Mehrotra R., Yadav K (2022)
Breast cancer in India: Present scenario and the challenges ahead. World J
Clin Oncol 13: 209-218. [Crossref]
2. Rivera E, Gomez H (2010)
Chemotherapy resistance in metastatic breast cancer: the evolving role of
ixabepilone. Breast Cancer Res 12 Suppl 2: S2. [Crossref]
3. Perou CM, Sorlie T, Eisen MB,
van de Rijn M, Jeffrey SS et al. (2000) Molecular portraits of human breast
tumors. Nature 406:747-752. [Crossref]
4. Lewis Wambi JS, Jordan VC
(2005) Treatment of postmenopausal breast cancer with Selective Estrogen
Receptor Modulators (SERMs). Breast Dis 24: 93-105. [Crossref]
5. Krishna BM, Chaudhary S.,
Mishra DR, Naik SK, Suklabaidya S et al. (2018) Estrogen receptor α dependent
regulation of estrogen related receptor β and its role in cell cycle in breast
cancer. BMC cancer, 18: 607. [Crossref]
6. Sun P, Wei L, Denkert C,
Lichtenegger W, Sehouli J (2006) The orphan nuclear receptors, estrogen
receptor-related receptors: their role as new biomarkers in gynecological
cancer. Anticancer Res 26: 1699-1706. [Crossref]
7. Zhang Z, Chen K, Shih JC, Teng
CT (2006) Estrogen-related receptors-stimulated monoamine oxidase B promoter
activity is down-regulated by estrogen receptors. Mol Endocrinol 20 7:
1547-1561. [Crossref]
8. Festuccia N, Owens N, Navarro P
(2018) Esrrb, an estrogen-related receptor involved in early development,
pluripotency, and reprogramming. FEBS lett 592: 852-877. [Crossref]
9. Yu S, Wong YC, Wang XH, Ling
MT, Ng CF et al. (2008) Orphan nuclear receptor estrogen-related receptor-beta
suppresses in vitro and in vivo growth of prostate cancer cells via
p21(WAF1/CIP1) induction and as a potential therapeutic target in prostate
cancer. Oncogene 27: 3313-3328. [Crossref]
10. Fernandez AI, Geng X, Chaldekas
K, Harris B, Duttargi A et al. (2020) The orphan nuclear receptor
estrogen-related receptor beta (ERRβ) in triple-negative breast cancer. Breast
Cancer Res Treat 179: 585-604. [Crossref]
11. Estrogen-related receptor beta.
Wikipedia.
12. Divekar SD, Tiek DM, Fernandez
A, Riggins RB (2016) Estrogen-related receptor β (ERRβ) - renaissance receptor
or receptor renaissance? Nucl Recept Signal 14: e002. [Crossref]
13. Zhou W, Liu Z, Wu J, Liu JH.,
Hyder SM et al. (2006) Identification and characterization of two novel
splicing isoforms of human estrogen-related receptor beta. J Clin Endocrinol
Metab 91: 569-579. [Crossref]
14. Huss JM, Garbacz WG, Xie W
(2015) Constitutive activities of estrogen-related receptors: transcriptional
regulation of metabolism by the ERR pathways in health and disease. Biochim
Biophys Acta 1852: 1912-1927. [Crossref]
15. Vanacker JM, Pettersson K,
Gustafsson JA, Laudet V (1999) Transcriptional targets shared by estrogen
receptor-related receptors (ERRS) and estrogen receptor (ER) α, but not by ERβ.
EMBO J 18: 4270-4279. [Crossref]
16. Sengupta D, Bhargava DK, Dixit A, Sahoo
BS, Biswas S et al. (2014) ERRβ signalling through FST and BCAS2 inhibits
cellular proliferation in breast cancer cells. Br J Cancer 110:
2144-2158. [Crossref]
17. Zabkiewicz C, Resaul J, Hargest
R, Jiang WG, Ye L (2017) Increased Expression of Follistatin in Breast cancer
Reduces Invasiveness and Clinically Correlates with Better Survival. Cancer
Genomics Proteomics 14: 241-251. [Crossref]
18. Heckler MM, Riggins RB (2015)
ERRβ splice variants differentially regulate cell cycle progression. Cell
Cycle 14: 31-45. [Crossref]
19. Tiek DM, Khatib SA, Trepicchio
CJ, Heckler MM, Divekar SD et al. (2019) Estrogen-related receptor β activation
and isoform shifting by cdc2-like kinase inhibition restricts migration and
intracranial tumor growth in glioblastoma. FASEB J 33: 13476-13491. [Crossref]
20. Bombail V, Collins F, Brown P,
Saunders PT (2010) Modulation of ER alpha transcriptional activity by the
orphan nuclear receptor ERR beta and evidence for differential effects of long-
and short-form splice variants. Mol Cell Endocrinol 314: 53-61. [Crossref]
21. Schüler Toprak S, Weber F,
Skrzypczak M, Ortmann O, Treeck O (2021) Expression of estrogen-related
receptors in ovarian cancer and impact on survival. J Cancer Res Clin Oncol
147: 2555-2567. [Crossref]
22. Kumari K, Adhya AK, Rath AK,
Reddy PB, Mishra SK (2018) Estrogen-related receptors alpha, beta and gamma
expression and function is associated with transcriptional repressor EZH2 in
breast carcinoma. BMC Cancer 18: 690. [Crossref]
23. Xia X, Hu T, He X, Liu Y, Yu C et al. (2023) Neddylation of HER2 Inhibits its Protein Degradation and promotes Breast cancer Progression. Int J Biol Sci 19: 377-392. [Crossref]
24. Naik SK, Lam EWF, Parija M, Prakash S, Jiramongkol Y et al. (2020) NEDDylation negatively regulates ERRβ expression to promote breast cancer tumorigenesis and progression. Cell Death Dis 11: 703. [Crossref]
