Multidisciplinary Management of Intrathoracic Goiter: A Case Report
A B S T R A C T
Introduction: Intrathoracic goiters are associated with compression of nearby structures, triggering severe compressive symptoms. Total thyroidectomy is the gold standard to treat these cases.
Case Presentation: A patient with a huge intrathoracic goiter suffering from compressive symptoms underwent a thorough clinical, functional and imaging assessment and underwent total thyroidectomy in an Endocrino-Metabolic surgical referral center; after the surgery she suffered from transient hypocalcaemia but was discharged without major complications and continued periodical endocrinological follow-up.
Conclusion: Management of intra-thoracic goiter requires a multidisciplinary approach of a skilled team both pre, during and after surgery to maximize the safety and efficacy of the procedure and reduce or promptly manage surgical or medical complications.
Keywords
Intrathoracic goiter, total thyroidectomy, neuromonitoing
Introduction
Goiter is defined as an abnormal enlargement of the thyroid gland and could be associated to different descriptions: diffuse or nodular, toxic, eu- or hypothyroid, associated or not with thyroid autoimmunity. Iodine deficiency is the most common cause of goiter worldwide, but several factors including genetics, hypothyroidism, smoking or goitrogens (e.g., cassava) increase the risk of goiter development. Autoimmune thyroid diseases (such as Hashimoto or Graves thyroiditis) and thyroid malignancies are often associated with goiter. Rare causes of goiter are also TSH-producing pituitary tumor and thyroid hormone resistance [1]. Goiter usually lies in the cervical region (85-90% of cases), but in a minority of cases it could be intrathoracic (ITG) if invading the thoracic inlet (10-15% of cases). ITGs are defined partial ITG, when at least 50% of thyroid tissue lies in the mediastinal space, and total ITG, when goiter lies completely below the sternal notch without evidence of thyroid enlargement in the neck. ITGs are retrosternal if located anteriorly to mediastinum, or retrotracheal if located posteriorly [2].
Unlike cervical goiters, ITGs are often symptomatic, yielding compression of nearby structures such as upper digestive and airway tract, nerves and vessels, triggering dyspnea, orthopnea, stridor and, less frequently, superior vena cava syndrome [3]. Medical therapy, levothyroxine in case of hypothyroidism or iodine replacement in areas of iodine deficiency, is of moderate utility, resulting at best in partial volume reduction. Radioactive iodine ablation has been associated with a 40–60% reduction in goiter volume within two years of therapy, however it could be complicated by an initial swelling, increasing the risk of airway compression [1]. Surgery, on the other hand, offers the possibility of a complete treatment, even if surgical complications such as recurrent laryngeal nerve injury, hypocalcaemia, and haemorrhage could develop more frequently in large ITGs than in small cervical goiters [4]. The choice is, if feasible on the surgical and clinical point of view, to perform total thyroidectomy, since subtotal thyroidectomy yields a high risk of re-surgery, increasing surgical complication risks. For these reasons, total thyroidectomy with cervical approach, intraoperative neurophysiologic monitoring (IONM) assistance and the use of high energy tools for haemostasis and dissection, is the treatment of choice to maximize the treatment efficacy mitigating the associated risks.
Case Presentation
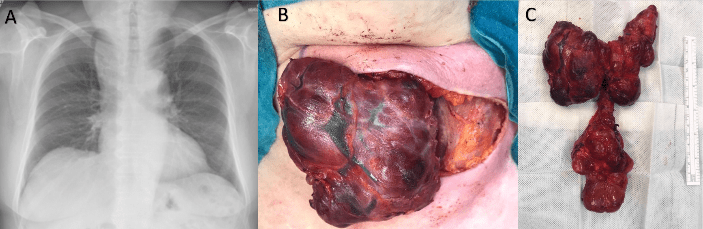
A 72 years-old female patient was admitted to our endocrinology outpatient clinic for mild hypothyroidism: TSH 11 µU/mL (n.v. 0.3-4.5) and fT4 7 pg/mL (n.v. 7.5-19). She had a history of subtotal thyroidectomy 30 years before for a benign goiter but did not perform any endocrinologic follow-up in the last decades. At physical examination, we appreciated a large multinodular goiter, with clear mediastinic extension, partially palpable at swallowing. Pemberton’s sign was positive bilaterally. Thyroid ultrasound revealed bilateral thyroid complex nodules without suspicious signs and identified a further nodular thyroid portion in the mediastinum, only partially detectable at swallowing. The patient complained of initial dyspnea in supine position, and worsening dysphagia for both liquids and solids. We treated the patient with levothyroxine 1 µg/kg/die, starting with lower doses until reaching euthyroid state, and completed the investigations with barium swallow and chest X-Ray examinations confirming the presence of a large intrathoracic goiter determining a slight tracheal deviation and mild oesophagus compression (Figure 1A).
Figure 1: Trilobular intrathoracic goiter. Slight tracheal deviation (A) Right thyroid lobe exposure (B) Resected thyroid gland weighting 240 g and presenting a particular trilobular shape with intramediastinic extension (C).
Considering the severity of symptoms referred by the patient, after collegial discussion with surgeons and in agreement with the patient’s will, we admitted the patient in our Endocrino-Metabolic Surgery Department to perform total thyroidectomy. No pre-operative fine-needle aspiration biopsy was scheduled, because of the presence of multiple and confluent nodules and the thyroid extension below the sternum. Pre-operative laboratory tests confirmed no contraindication to surgery. The patient underwent an IONM (Intra Operative Nerve Monitoring) procedure performed with ultrasound device for haemostasis and dissection. We started surgery with a 12-cm Kocher’s cervicotomy incision on the previous one, superior and inferior musculocutaneous flap preparation and dissection without transection of the anterior neck muscles According with the previous classification, we identified a partially intrathoracic and retrosternal goiter. We primarily performed surgery on the most voluminous right thyroid lobe (Figure 1B). The first step was the dissection from muscles, carotid artery and jugular vein, exposing the vagal nerve for the V1 stimulation, which was > 1200 µV. The second step was the recurrent laryngeal nerve intraoperative monitoring (NIM) assisted detection and visual identification, finding a R1 stimulation > 500 µV, allowing us to continue the procedure; identification and dissection of superior and inferior parathyroid glands and section of the upper and lower thyroid artery were performed with high energy device and we completed the right lobectomy maintaining RLN visualization and NIM. R2 and V2 signal were adequate (>500 µV), so we could safely perform the contralateral lobectomy. The left thyroid lobe was bound to an atypical “third posterior lobe”, which lay completely in the mediastinum (Figure 1C). The procedure was performed using the same cervical access, without complications. Two 15-Fr drains were positioned before skin suture. The procedure, performed by two skilled thyroid surgeons and a trained resident, took 146 min with no blood loss.
Post-operative laryngoscopy was normal, except for a mild left chordal edema. The patient showed no fever or pathological voice alterations, hence she was discharged after 4 days. Post-operative rehabilitation was complicated by symptomatic mild hypocalcemia effectively treated with 2 g calcium and 0.75 µg of calcitriol administration, which spontaneously healed after 2 months. After surgery, the patient also incremented gradually levothyroxine replacement therapy (reaching the dose of 1.3 µg/kg/die). Final histological result was consistent with thyroid nodular hyperplasia and adenomatous characteristics. Resected thyroid gland weighted 240 g and presented a particular trilobular shape with intramediastinic extension (Figure 1C). Follow-up examination showed satisfactory thyroid hormone replacement and neck post-operative ultrasound did not identify thyroid remnants.
Discussion
This clinical case represents a paradigmatic example of high complexity in thyroid surgery, highlighting a number of management clues. In our Institution, we follow precise recommendations to perform a safe-and-effective surgical procedure, derived both from international guidelines and internal protocols:
1. prior to surgery, patients should undergo a thorough clinical, functional and imaging assessment.
2. thyroid nodules fine-needle-aspiration (FNA) could be a useful tool to characterize the goiter offering important information for surgical and clinical management. Unfortunately, in ITGs the position and the type of goiter often makes this procedure unfeasible. However, since total thyroidectomy is the treatment of choice in ITGs, regardless the nature of goiter nodules, in most cases we can consider FNA not mandatory.
3. surgery indication and patient preparation should be discussed with the attending endocrinologist.
4. thyroidectomy for partial or total intrathoracic goiter must be performed by an expert surgeon (at least 100 thyroid surgical interventions/year) in a “high volume” centre.
5. every procedure must be IONM assisted for a safe nerve sparing, following the International NeuroMonitoring Study Group guidelines [5].
6. every patient has to undergo a pre-operatory and post-operatory laryngoscopy for a correct vocal cord mobility evaluation and management [5].
7. surgery should be performed with high energy haemostasis devices to control intra-operative bleeding and reduce the post-operative bleeding risk [6].
8. we recommend placing the patient in an intensive observation recovery room for 2 to 4 hours before returning to hospital ward.
9. serum calcium screening 24 and 48 hours after surgery is mandatory to monitor parathyroid function.
10. during hospitalization, endocrinologist consultancy should be requested to evaluate the best levothyroxine replacement therapy dose, assess clinical signs and symptoms of hypocalcaemia, and provide prompt substitution if needed.
11. after hospitalization, patient should undergo surgical and endocrinological controls to assess wound integrity, possible complications and the correct hormonal replacement.
12. thyroid ultrasound should be performed 3 months after surgery to verify the presence of possible thyroid remnants.
Conclusion
The surgical management of complex ITGs requires a multidisciplinary approach determining a careful pre-operative planning, the implementation of intra-operative modern and skilled techniques and a rigorous post-operative evaluation.
Article Info
Article Type
Case ReportPublication history
Received: Fri 20, Sep 2019Accepted: Tue 08, Oct 2019
Published: Thu 31, Oct 2019
Copyright
© 2023 Maria Laura Tanda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJSCR.2019.02.06
Author Info
Davide Inversini Jessica Sabatino Maria Laura Tanda Matteo Annoni Silvia Ippolito
Corresponding Author
Maria Laura TandaDepartment of Endocrinology, Insubria University, Varese
Figures & Tables
References
- Hegedus L, Bonnema SJ, Bennedbaek FN (2003) Management of simple nodular goiter: current status and future perspectives. Endocr Rev 24: 102-132. [Crossref]
- Perincek G, Avci S, Celtikci P (2018) Retrosternal Goiter: A couple of classification methods with computed tomograpy findings. Pak J Med Sci 34: 1494-1497. [Crossref]
- Yang X, Gao T, Shi R, Zhou X, Qu J et al. (2014) Effect of iodine excess on Th1, Th2, Th17, and Treg cell subpopulations in the thyroid of NOD.H-2h4 mice. Biol Trace Elem Res 159: 288-296. [Crossref]
- Doulaptsi M, Karatzanis A, Prokopakis E, Velegrakis S, Loutsidi A et al. (2019) Substernal goiter: Treatment and challenges. Twenty-two years of experience in diagnosis and management of substernal goiters. Auris Nasus Larynx 46: 246-251. [Crossref]
- Wu CW, Dionigi G, Barczynski M, Chiang FY, Dralle H et al. (2018) International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018. 128: S18-S27. [Crossref]
- Dionigi G, Wu CW, Kim HY, Liu X, Liu R et al. (2016) Safety of energy based devices for hemostasis in thyroid surgery. Gland Surg 5: 490-494. [Crossref]
