Mucinous Adenocarcinoma Associated with Fistula in an 87-Year-Old Managed with Neoadjuvant Chemoradiation and ELAPE at Tata Memorial Hospital, in Mumbai
A B S T R A C T
Colorectal cancer is the third most diagnosed cancer and the fourth leading cancer-related death worldwide. Mucinous adenocarcinoma associated with anal fistula is a rare variant of adenocarcinoma, presents with delayed diagnosis, locally advanced, low nodal, and no distant metastasis. Adenocarcinoma associated with fistula (ACAF) is rare, has delayed diagnosis and poor prognosis but can be managed with neoadjuvant chemoradiation (NACRT) and complete curative resections with reconstruction by V-Y advancement cutaneous flap.
Keywords
Colorectal cancer, colorectal mucinous adenocarcinoma, anal cancer, adenocarcinoma associated with anal fistula, anal fistula
Introduction
Colorectal cancer is the third most diagnosed cancer and the fourth leading cancer-related death worldwide, affecting men and women anaesthesia [1, 2]. The commonest type of colorectal cancer is adenocarcinoma, with mucinous and non-mucinous subtypes characterised based on mucinous components tumor content [1]. Mucinous adenocarcinoma, first described by Parham in 1923, accounts for 10-20% of colorectal carcinoma [3, 4]. It affects mainly females, presents at a young age, and has three distinct subtypes based on the classification by the World Health Organization as mucinous adenocarcinoma, signet cell carcinoma, and mucin containing the rectal tumor [1, 3, 5, 6].
The first type is mucinous colorectal carcinoma with greater than 50% extracellular mucin and accounts for 5-15% of colorectal carcinoma, while the second type is signet cell carcinoma, is rare, in which the mucin is only intracellular [3, 5, 6]. The third type is mucin-containing carcinoma, in which acellular mucin pools develop as a response to therapy and is considered an excellent prognostic response to neoadjuvant chemoradiation [3, 7]. Anorectal adenocarcinoma is rarely, at 1% of colorectal carcinoma and can be associated with anal fistula [1, 8]. Adenocarcinoma associated with fistula (ACAF), first described by Roser in 1923, is rare, arises in a chronic anal fistula, and accounts for 6.9% of anorectal carcinoma [1, 8, 9]. Generally, ACAF has a worse prognosis with the diagnosis made at an advanced stage, greater risk of nodal and peritoneal metastases, and reduced overall survival [2, 7].
Standard treatment to locally advanced ACAF is neoadjuvant chemoradiation, to improve resectability with tumor-free margins, by curative complete resections and flaps for reconstruction [1, 8]. Neoadjuvant chemoradiation reduces local recurrence and lowers toxicity to preoperative chemoradiation compared to adjuvant therapy, though the benefit is controversial [1, 8]. This paper aims to show how ACAF was managed using neoadjuvant therapy, extralevator Abdominal Perineal Excision (ELAPE), and reconstruction with V-Y cutaneous advancement flap at Tata Memorial Hospital in Mumbai, India.
Case Presentation
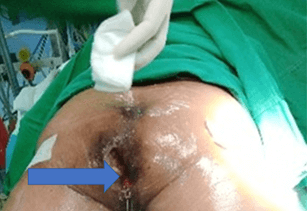
An 81-year-old gentleman presented with complaints of pain, perianal discharge, and weight loss of 10 months duration. Physical examination revealed good general condition with a performance score of 1, no anaemia, or lymphadenopathy. His abdominal examination was unremarkable. Digital rectal examination revealed an ulcerative lesion in the left gluteal region measuring 4cm by 5cm with bloody, mucoid discharge, as shown in (Figure 1).
Figure 1: Arrow points to ACAF in an 87-year-old patient.
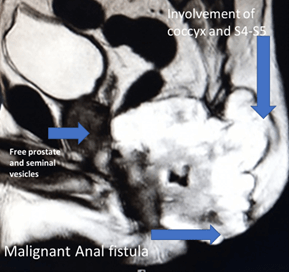
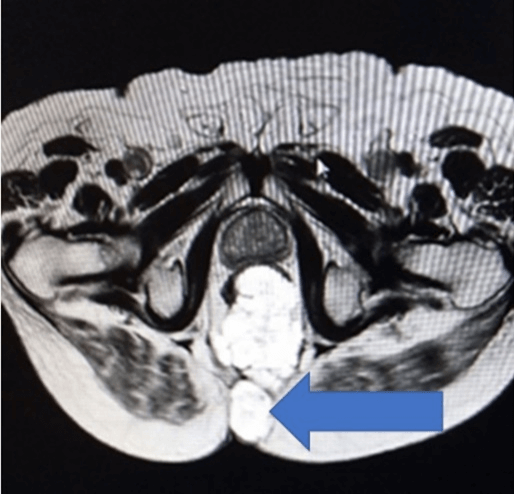
A complete colonoscopy could not be performed as scope could not be negotiated beyond the tumor. The Anorectal lesion was biopsied. The carcinoembryonic antigen (CEA) levels were 10.26ng/ml (indicating advanced disease) and 12.6g/dl haemoglobin. The MRI revealed a large lower rectal, perianal mucinous tumor anal fistula at 5’0 clock: levator muscles, ischiorectal tissue, coccyx, fourth and fifth sacral vertebra, bilateral ischioanal fat pad, and abutting left obturator fascia (cT4a), as shown in (Figures 2 & 3). No significant lymph nodes were noted (cN0). Contrast-enhanced CT Scan did not detect any distant metastasis (cM0). Biopsy of the perianal mass revealed Moderately differentiated mucinous adenocarcinoma with American Joint Committee on Cancer (AJCC) stage IVa (locally advanced).
Figure 2: MRI image showing locally advanced ACAF spread to sacrum and anal sphincter.
Figure 3: Arrow showing malignant fistula at 5’O clock.
Treatment plan advised by multidisciplinary team (MDT) (involving a colorectal surgical oncologist, radiation oncologist, radiologist, medical oncologist, and gastroenterologist) meeting, advised neoadjuvant chemoradiation therapy and surgery after. The patient received a well-tolerated course of neoadjuvant short course radiation therapy (NACTRT) of 25Gy of radiation in 5 fractions; and reviewed afterward by MDT, which recommended extralevator abdominal perineal excision (ELAPE) with reconstruction by a V-Y advancement flap.
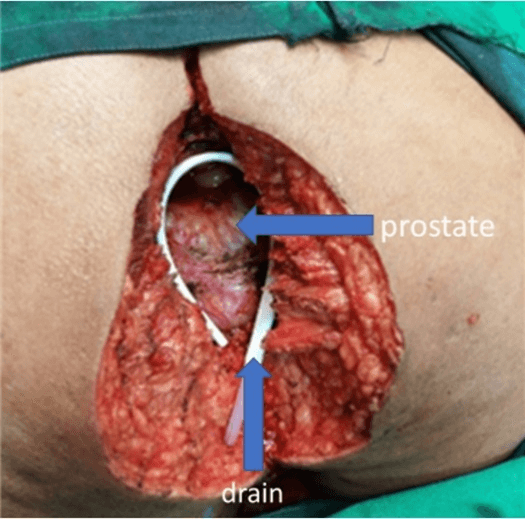
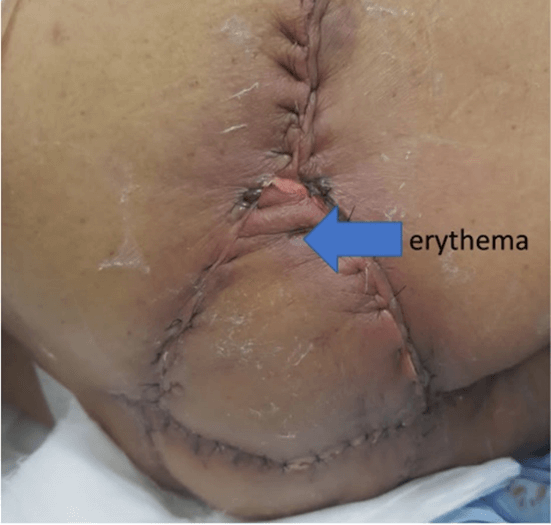
Laparoscopic resection of the rectum (with separation of the bladder, prostate, and seminal vesicles from the rectum) was done. The left colon conduit's mobilisation was done in the supine position — the lymphatics along the inferior mesenteric artery excised with the rectum. Rectum was divided and stapled at a point distal to the origin of the first sigmoid branch, with permanent Hartmann’s colostomy and closure of ports done. In the second part of the procedure, the complete perineal resection was done in a prone position, as shown in (Figure 1). The sacral cut was taken between S3 and S4 and excised together with the specimen en bloc. The defect shown in (Figure 4) was closed by a V-Y advancement cutaneous flap by a team of reconstruction surgeons. The tumor staging after surgery was cT4, cN1, and cM0. There was erythema due to tension in the flap's suture line, as shown in (Figure 5), which was released. After that, the rest of the postoperative recovery was uneventful. The final pathological report was pT3, pN0, and pM0.
Figure 4: Showing perianal defect after ELAPE in the 87-year-old patient placed in prone position.
Figure 5: Day 3 post V-Y advancement cutaneous flap closure of perianal defect. Erythema developed due to tension in suture line. Suture release was done. Erythema resolved before discharge.
Discussion
Adenocarcinoma originating in a chronic anal fistula is rare and often diagnosed late, in advanced condition, because initial clinical features are difficult to distinguish from benign anal fistula [1, 8]. Therefore, considering clinical details with attention given to duration, non-responsiveness to previous therapy (fistulectomy by seton suture or open fistulectomy) should raise a high index of suspicion [8]. Rosser established criteria for the diagnosis of adenocarcinoma associated with fistula that it should precede carcinoma by ten years, internal opening in the anal canal, fistula tract be through the tumor and external opening on the tumor itself [8, 10]. Our elderly patient had ACAF, delayed presentation but symptom duration of 10 months is shorter from what Rosser described, suggesting aggressiveness of the tumor [10]. Mucinous histology, associated with young age at presentation, was diagnosed in a male, elderly patient [11]. Though not the typical age of presentation but the lesion was advanced.
Shinde et al. have published their experience of treating three locally advanced histological groups of adenocarcinomas using long course NACRT followed by re-evaluation after 4-6 weeks for curative surgical treatment at Tata Memorial Hospital [12]. The three histological groups were composed of signet cell carcinoma, mucinous adenocarcinoma, and non-signet cell adenocarcinoma. Comparing the three groups for tumor response rate, recurrence rate, disease free survival (DFS), and overall survive (OS) post NACRT revealed that with improved surgical technique and adequate CRM, there was no DFS and OS. The complete pathological response rate was 27% in mucinous adenocarcinoma, which does not reduce mucinous adenocarcinoma tumor volume, but mucin becomes acellular [12]. However, our patient was elderly and hard, locally advanced mucinous adenocarcinoma. Short course NACRT was given and after re-evaluation, offered curative surgery, which others documented [12].
There is evidence that NACRT, followed by curative surgery with complete excision, can result in good oncological outcomes with good 5-year survival. NCCN guidelines recommend neoadjuvant chemoradiotherapy followed by TME as the standard treatment for locally advanced adenocarcinoma of rectum irrespective of histological subtype [13]. Nekajima et al. reported a 5-year survival of 74% and 16% for those that received curative and non-curative surgery, respectively [14]. Locally, the division of colorectal surgery at Tata Memorial hospital have published their experience in a retrospective study by V. D. Pai et al., that reported management of locally advanced ACAF with the administration of NACRT, routine use of MRI to stage the patient before surgery, and ELAPE with reconstruction surgery by a V-Y advancement flap as shown in, with good results [8]. They report that such results are possible with NACRT and MRI's liberal use before surgery [8]. However, surgeon competence and experience are critical to negative tumor resections that offer curative surgery, which has also been reported by other studies [8].
Colonoscopy is mandatory to rule out the presence of metachronous disease, but in situations where the lesion is constricting and circumferential, negotiating the scope may not be possible, as in our case [1, 6]. Arya et al. have stated that Tata Memorial Hospital is a tertiary center that receives patients with a higher T stage and patients with locally advanced anal tumors [15]. Only 1 % of patients underwent a colonoscopy with endoscopic ultrasound. CECT and diagnostic laparoscopy were performed to rule out metastases in our patients. CEA and MRI (hyperintense T2) imaging are not accurate to be relied on for diagnosis; MRI helps to detect mucinous component and helps with the differential diagnosis but does not downstage tumor to the persistence of mucin and development of acellular mucin component that can result in overstating, as a response to NACRT [1]. There was no change in the patient’s reduction in CEA and tumor regression on MRI, as reported, the lack of microvasculature resulting in reduced blood supply in mucin results in an inadequate response [6].
Conclusion
Our patient treatment outcome agrees with our previous publication and other authors showing that ACAF is locally advanced, with a low incidence of nodal metastasis and no distant metastasis. There is a role for the use of NACRT before surgery to attain negative margins.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Wed 18, Nov 2020Accepted: Sat 28, Nov 2020
Published: Tue 08, Dec 2020
Copyright
© 2023 Avanish Saklani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GSCR.2020.02.09
Author Info
Seke Manase Ephraim KAZUMA Vivek Sukumar Avanish Saklani
Corresponding Author
Avanish SaklaniProfessor and Consultant Colorectal Surgeon and Head of Department of Colorectal Surgical Oncology, Tata Memorial Hospital, Parel, Mumbai, India
Figures & Tables
References
- Luo C, Cen S, Ding G, Wu W (2019) Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun 39: 1-13. [Crossref]
- Ott C, Gerken M, Hirsch D, Fest P, Feigl SF et al. (2018) Advanced Mucinous Colorectal Cancer: Epidemiology, Prognosis, and Efficacy of Chemotherapeutic Treatment. Digestion 98: 143-152. [Crossref]
- Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G et al. (2005) Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 16: 1305-1310. [Crossref]
- Hu X, Li YQ, Li QG, Ma YL, Peng JJ et al. (2018) Mucinous Adenocarcinomas Histotype Can Also be a High-Risk Factor for Stage II Colorectal Cancer Patients. Cell Physiol Biochem 47: 630-640. [Crossref]
- Shin US, Yu CS, Kim JH, Kim TW, Lim SB et al. (2011) Mucinous rectal cancer: Effectiveness of preoperative chemoradiotherapy and prognosis. Ann Surg Oncol 18: 2232-2239. [Crossref]
- Wnorowski AM, Menias CO, Pickhardt PJ, Kim DH, Hara AK et al. (2019) Mucin-containing rectal carcinomas: Overview of unique clinical and imaging features. AJR AM J Roentgenol 1-9. [Crossref]
- Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R et al. (2009) Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 100: 881-887. [Crossref]
- Pai VD, Jatal S, Engineer R, Ostwal V, Saklani AP (2015) Multidisciplinary management of colorectal adenocarcinoma associated with anal fistula: An Indian series. Colorectal Dis 17: O240-O246. [Crossref]
- Pai VD, Jatal S, Ostwal V, Engineer R, Arya S et al. (2016) Multivisceral resections for rectal cancers: Short-term oncological and clinical outcomes from a tertiary-care center in India. J Gastrointest Oncol 7: 345-353. [Crossref]
- Rosser C (1934) The relationship of fistula-in ano to cancer of the anal canal. Trans Am Proc Soc 35: 65-71.
- Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW et al. (2012) Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: Analysis from the National Cancer Data Base. Ann Surg Oncol 19: 2814-2821. [Crossref]
- Shinde RS, Katdare N, Kumar NAN, Bhamre R, Desouza A et al. (2018) Impact of histological subtype on treatment outcomes in locally advanced rectal adenocarcinoma treated with neoadjuvant chemoradiation. Acta Oncol 57: 1721-1723. [Crossref]
- Blocks NE (2020) Anal Carcinoma.
- Nakajima K, Kobayashi AKT (2010) Carcinoma associated with anal fistula: a clinicopathologic study in 15 patients. Jpn Soc Coloproctol 2010: 346-358.
- Arya S, Das D, Engineer R, Saklani A (2015) Imaging in rectal cancer with emphasis on local staging with MRI. Indian J Radiol Imaging 25: 148-161. [Crossref]
