Modulation of Oral Biofilm and Immune Response Associated to Mucosa with Probiotic Bacteria as a Potential Approach in the Prevention of Dental Caries: A Systematic Review
A B S T R A C T
Background: A variety of approaches have been developed for the control of dental caries, a pathology with high incidence and prevalence worldwide. The use of probiotic strains for the modulation of dental biofilm in the prevention of caries has been studied, but the available evidence shows varied methodologies; and the strains tested differ from one study to another.
Objective: To analyse through a systematic review of clinical trials, the efficacy of using probiotic strains to prevent dental caries.
Methods: A search was made in the scientific bases PubMed, Cochrane and Science Direct, prioritizing randomized double and triple-blind clinical trials from 2010 to 2020, including a total of 20 studies to be analysed. The selection criteria were consistent with the Preferred Reporting Items for Systematic Reviews protocol.
Results: Within the studies, different types of probiotic bacteria were analysed, dominating Lactobacillus Paracasei. While most clinical trials show a favorable response in terms of a significant reduction of Streptococcus mutans in the oral microbiota, very few studies evaluated salivary pH and sIgA levels.
Conclusion: The heterogeneity of the studies analysed and the multifactorial nature of dental caries do not allow us to ensure that probiotic therapy is completely effective in preventing this pathology. Although probiotic therapy can help by regulating the microbiological factor, there are other determinants that can favor the development of caries and that are barely approached in their relationship with bacteriotherapy. Future studies that homogeneously evaluate the use of probiotics could give us a clearer idea of their effectiveness.
Keywords
Oral microbiota, dental caries, probiotics, Streptococcus mutans
Introduction
Dental caries is a chronic disease and a global public health problem, imposing a large economic and health burden internationally; a wide variety of approaches to its control have been developed and applied, with varying degrees of success [1, 2]. According to Global Burden data on oral diseases, untreated caries in permanent teeth affects about 2.5 billion people around the world, making it the most prevalent oral pathology above others [3]. Caries also greatly affects primary teeth, with a prevalence of 17% in children with 2 years old, 48% at age 4 and rising to 70% in children aged 6 according to the World Health Organization (WHO). During childhood, this disease attacks more aggressively and can progress to tooth loss if is not treated in time [1, 4, 5]. Early childhood caries increases the risk of tooth decay in permanent teeth; it is also associated with a 3-7 month delay in the development of permanent teeth and its late maturation affects occlusion and can lead to problems with eating, speech, appearance, and behaviour [5-7]. This is a multifactorial disease; however, the main causal agent is represented by pathogenic acidic/aciduric microorganisms such as Streptococcus mutans, accompanied by dysbiosis in the oral cavity [8-10].
The negative effects of dental caries on health are cumulative, from childhood to adulthood, nevertheless, it is a preventable pathology [11, 12]. Proper oral hygiene is essential to prevent its development [13]. Nowadays the most commonly used prophylactic chemical agents inhibit bacterial growth and biofilm formation. However, these chemical agents also eliminate the oral microbiota, altering its balance and potentially affecting oral health [4, 14]. Although several medicines can inhibit the pathogenic microbiota, none has been able to successfully prevent the proliferation of residual strains, which is why the selective inhibition of pathogenic microorganisms through the use of probiotic strains has been studied recently [4]. The definition of “probiotics” has been adopted by the World Health Organization as: “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” [15]. These have been used historically for gastrointestinal and immunological treatments and are now widely studied as preventive therapy in oral health [16-18]. The main mechanism of action is based on enhancing the commensal microbiota and preventing colonization by true pathogenic microorganisms [16]. The present study aims to analyse, through a systematic review of different randomized and controlled clinical trials, the efficacy of probiotic bacteria for potential preventive therapy of dental caries by modulating biofilm and stimulating the immune response associated to mucosa.
Data collected from most studies show that probiotics produce a significant reduction in the colonization density (expressed by the count of colony forming units/ml/cm2) of Streptococcus mutans in short and medium term; other studies demonstrated an increase in salivary pH and secretory IgA levels (sIgA), which provide a positive effect in the prevention of dental caries. However, the evidence for the immunomodulatory effect of probiotic strains, measured by salivary pH and/or salivary buffer capacity, in addition to sIgA levels, is very poor. Therefore, more high-quality studies that can be reproduced are needed.
Materials and Methods
This section was planned following the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-analyses system (PRISMA criteria) [19].
I Matter in Question
Probiotics prevent dental caries via modulation of the biofilm and mucosa associated immune response.
II Eligibility or Inclusion Criteria
Randomized, blinded, controlled clinical trials that evaluate the effect of probiotic strains on Streptococcus mutans count, salivary pH, salivary buffer capacity, and sIgA levels.
III Exclusion Criteria
Letters to the editor, review articles, association articles, in vitro experimental studies, unblinded randomized studies and articles that do not involve the subject matter.
IV Search Strategy
Three digital databases, PubMed, Science Direct and Cochrane, were selected for the collection of scientific articles published during the period 2010-2020. Four reviewers conducted the search strategy to identify eligible studies. MeSH terms in all fields were used for the search in order to maximize the search during the investigation [20]. The search strategies defined for the databases previously described were probiotics “AND” caries, and probiotics “AND” dental biofilm. Restrictions were imposed on publication data and access to the scientific article; only English language publications were considered. Articles available in more than one database were considered only once.
V Selection of Studies
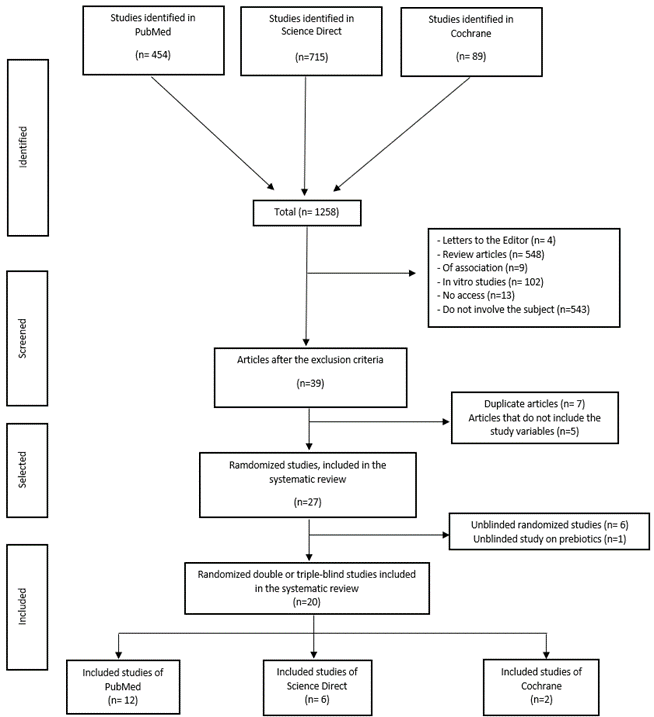
Initially 27 randomized and controlled clinical trials were selected for the evaluation of salivary pH, CFU/ml values of Streptococcus mutans (Sm) and sIgA, however, in view of little evidence about prebiotics and their modulation of biofilm it was decided to exclude these studies. Finally, 20 articles were included for the systematic review. Figure 1 details the flowchart for the selection of studies.
VI Data Register
Microsoft Excel database was used for the processing of the data. The qualitative variables to be interpreted were: significant reduction (respect to the control group) in the Sm count, significant increase in the salivary sIgA level, significant increase of salivary pH, salivary buffer capacity; the age range is from 4 months to 73 years old, including both male and female individuals.
VII Results Prioritization
Data analysis was done through qualitative description.
VIII Bias Risk
Not assessed due to variability in methodology and plurality of objectives of each study. However, in order to increase the quality of evidence, of all articles related to the topic and obtained, we selected for full reading, only those that incorporated double and triple blinding. Discarding the clinical trials with simple blinding or without blinding.
Figure 1: Flowchart that shows the methodology used in the selection of the studies that passed to the full analysis.
Results
The PubMed search provided 454 articles (2010 to 2020), ScienceDirect 715 articles (2010 to 2020) and Cochrane with 89 articles (2010 to 2020), with the keyword’s probiotics “AND” caries, and probiotics “AND” dental biofilm. After a thorough review in these databases, a total of 1258 articles were retrieved, of which 39 controlled and randomized studies were considered. Of these, 12 were excluded for not contributing with the variables chosen in this study and for being duplicated; an additional filtering of the 27 studies was carried out after reading the abstract and methodology, which determined the exclusion of 7 articles that studied prebiotics and were not blinding. A total of 20 articles were finally included in the systematic review: PubMed provided with 12 articles, Science Direct with 6 and Cochrane with 2 articles. The total number of participants in the different randomized, double and triple-blind clinical trials analysed was 2037, the sample size of the total trial’s ranges from 19 to 321 participants with an average of 102. According to the gender of the participants, a total of 1013 were males representing 52.6% and 913 females representing 47.4% of all the studies; it is worth mentioning that three studies do not mention a gender-disaggregated registration, the total number of participants in these was 111. According to the age of the participants, the total range was from 4 months to 73 years, the average being 12.6 years.
In this review, different probiotic species were encompassed, of which the most predominant were: Lactobacillus paracasei with 30%, followed by Lactobacillus rhamnosus 20%, Lactobacillus reuteri 15%, combined probiotics 10%, Bacillus coagulans 10%, and the remaining 15% were found as probiotics: Bifidobacteruim longum, Lactobacillus acidophilus, Bacteriocin, Bifidobacterium lactis, Lactobacillus casei, and Streptococcus salivarius. Our study determined that the treatment time range was 7 to 365 days, with an average treatment time of 89.32 days. In the analysed studies, the count of colony forming units (CFU) of S. mutans per ml or cm2 was made by cultures media; the salivary pH and the buffer capacity were measured with a digital laboratory pH meter or with pH test strips. On the other hand, the salivary levels of sIgA were determined by the ELISA method. Of the 20 studies collected, 19 (95%) evaluated the S. mutans count, 3 (15%) measured the sIgA levels and 6 (30%) evaluated the salivary pH and buffer capacity. Of the most found probiotic species, whose effect was evaluated in at least 3 of the 4 variables proposed in this analysis, were L. rhamnosus and L. paracasei. Meanwhile, L. reuteri was analysed in at least 2 of the 4 variables proposed in this review. Finally, the species L. paracasei was identified as the most effective in modulating the biofilm for caries prevention, because it significantly reduces the number of S. mutans, increases salivary pH and sIgA (Table 1).
Table 1: Distribution of probiotic species evaluated in terms of efficacy with respect to the control group, in all consensus studies.
|
Bacterial Species |
Nº of studies (%) |
Significant reduction in Sm (%) |
Significant increase in salivary pH/ buffer capacity |
Significant increase in salivary level of sIgA |
Average treatment time in days |
|
L. rhamnosus |
5 (25%) |
3 (60%) |
2 (40%) |
0 |
68,7 days |
|
L. paracasei |
6 (30%) |
5 (83,3%) |
1 (16,6%) |
1 (16,6%) |
128,5 days |
|
L. reuteri |
3 (15%) |
1 (33,3%) |
1 (33,3%) |
0 |
147,6 days |
|
L. acidophilus |
2 (10%) |
2 (100%) |
0 |
0 |
22 days |
|
L. casei |
1 (5%) |
1 (100%) |
0 |
0 |
57 days |
|
L. sporogenes |
1 (5%) |
1 (100%) |
0 |
0 |
30 days |
|
Bacillus coagulans |
2 (10%) |
1 (50%) |
0 |
0 |
10,5 days |
|
Bifidobacterium lactis |
1 (5%) |
1 (100%) |
0 |
0 |
14 days |
|
Streptococcus salivarius |
1 (5%) |
1 (100%) |
0 |
0 |
91,2 days |
Discussion
This study reviewed certain parameters that must be considered for approval of the use of probiotics in the prevention of dental caries, such as: 1) Reduction of key cariogenic bacteria such as Streptococcus mutans, 2) Increase in the pH and buffer capacity of saliva, and 3) Increase in the salivary level of secretory IgA (sIgA). The use of probiotics helps maintain health by providing balance to the gastrointestinal, genitourinary, and oral ecosystem; probiotics have been shown to have the ability to reduce the S. mutans count in saliva or dental plaque in the short term [21, 22]. In our study we found the same results, and we identified that Lactobacillus rhamnosus, Lactobacillus reuteri and Lactobacillus paracasei species were the most studied, being this last one the one with more positive effects in terms of significant decrease, relative to control group, in the number of CFU/ml/cm2 of S. mutans, as well as, significant increase in sIgA and salivary pH levels. Summarizing the results at a global level for all the probiotic species evaluated, in 14 of the 20 selected articles a significant reduction in the S. mutans count was identified, in 4 articles a significant increase in salivary pH/buffer capacity was identified and one demonstrated a significant increase in sIgA levels.
On the other hand, we can mention that probiotics show other advantages for general health since they improve the immune system; in addition, the vehicles by which they can be administered are various, such as: pills, milk, cheese, yogurt, ice cream, drops, powder, mouthwash, chewing gums, among others [21, 23]. Chewing gums with probiotics and sugar-free have been shown to reduce the acidogenicity of plaque and increase the remineralization of the enamel, improving salivary flow and oral health [22]. However, some disadvantages of probiotics have also been described, as they can cause adverse effects including upset stomach, diarrhea, headache, fever, and nausea or vomiting, according to the study by Teanpaisan et al. (2014) [24]. The main cause of dental caries is the accumulation of dental biofilm; in order to prevent this, it is important to eliminate bacterial plaque by mechanical removal through tooth brushing, however, there are chemical methods to control biofilm and as an alternative to these arise from the incorporation of probiotics that can be an excellent complement to brushing, and they are also considered safe for oral administration in humans [24-26].
The most widely used chemical solution for biofilm control is chlorhexidine, but its use may present some drawbacks such as dental pigmentation, taste alteration, hypersensitivity and stenosis of the parotid duct; that is why the use of probiotics could represent an advantage against weaknesses of certain chemical products [26, 27]. The antiplaque activity of probiotics is achieved by reducing bacterial adhesion to the tooth surface, inhibiting the growth and proliferation of pathogenic microorganisms, avoiding the formation of intercellular matrix and modifying the biochemistry of plaque to reduce the formation of cytotoxic products, obtaining a less pathogenic microbiota [25, 26, 28]. Jothika et al. (2015) mention that chlorhexidine varnish, gel and mouthwash reduce the level of S. mutans in saliva, inhibit plaque formation and increase salivary pH values, however, it can produce dental pigmentation when used for a long time [25, 29]. In the study by Shah et al. (2019), the group treated with probiotic mouthwash and the group with chlorhexidine obtained significantly reduced plaque index, compared to the control group, however, substantial improvement was observed in the gingival index than in the plaque indices with better results in the probiotic group [26].
Stensson et al. (2014), did not detect statistically significant differences for the plaque index between the control group and the probiotic group when using oral drops of Lactobacillus reuteri during the first year of life, however, 9-year-old children who had received perinatal supplements of L. reuteri since birth and during their first year were almost caries-free and had a lower prevalence of proximal caries [28, 30]. Matuq et al. (2020), report that probiotic tablets with L. reuteri and chlorhexidine (CHX) mouthwash significantly reduce the accumulation of plaque and S. mutans count and increase salivary pH values [25]. Statistically similar effects on plaque control were found in the trial by Jothika et al. (2015), however, their study used Lactobacillus acidophilus as a probiotic in mouthwash and was compared with CHX and sodium fluoride mouthwashes [29]. The antiplaque effect of L. reuteri strains in probiotic tablets can be attributed to their ability to prevent microorganisms from adhering and growing on the tooth surface, in addition to modifying the biochemistry of the biofilm [25, 28].
Regarding Lactobacillus acidophilus, Tahmourespour et al. (2011) and Jothika et al. (2015), clarify that its presence can cause a reduction in the adherence of streptococcal strains, mainly S. mutans [29, 31]. In our review, L. paracasei was the most widely used probiotic; and in the trial by Manmontri et al. (2020), this probiotic species reduces the number of cariogenic bacteria such as S. mutans in both saliva and dental plaque, and its reduction effect persists for at least 6 months after the discontinuation of the probiotic, this may be a consequence of the constant microbial modulation of L. paracasei SD1 and its ability to adhere to the epithelial cells of the oral mucosa [10]. In addition, other studies attribute the highest antimicrobial activity to L. paracasei, Lactobacillus plantarum, L. rhamnosus and L. salivarius, with L. paracasei being one of the commensal species of the genus Lactobacillus that has shown maximum interference activity against S. mutans, even in vitro [15].
In the study by Nishihara et al. (2014), 2 different strains of Lactobacillus were compared against an antibody obtained from the IgY-type egg yolk (Ovalgen DC), and Xylitol. In these groups, the changes in the density of colonization by Streptococcus mutans and anticariogenic commensal bacteria, increased salivary pH and buffer capacity were evaluated. Both, probiotic treatment and immunotherapy with Ovalgen DC were shown to reduce S. mutans levels, and increase salivary flow and pH, but without significant differences compared to xylitol treatment. However, of the 3 approaches, only the probiotic treatment demonstrated superiority in increasing colonization by commensal Lactobacillus species, and together with immunotherapy they demonstrated an increase in salivary buffer capacity, with significant differences over the group treated with xylitol. The study concluded that certain probiotic strains are more effective in preventing tooth decay than chemical plaque or biofilm control methods [32].
Reduction in the level of Streptococcus mutans colonization can be achieved by a vaccine, however, only few controlled and double-blind clinical studies have been published to evaluate active immunization strategies; and only open clinical studies have been conducted to evaluate the efficacy of passive immunization [33-36]. In the passive vaccines, antibodies are administered that distribute a specific protection, this significantly reduces the colonization of Streptococcus mutans, but they cannot be maintained, therefore they are less effective and require multiple applications to maintain the immunization [34, 37]. The results of controlled clinical trials on the efficacy of active immunization in the prevention of dental caries have been encouraging; however, the salivary responses were variable, transitory and low magnitude [38-42].
Randomized, double-blind phase 1 clinical trials conducted by Childers group demonstrate that active immunization with an antigenic preparation enriched in Streptococcus mutans glycosyltransferase (GSF) and administered by nasal route with liposomal coating is more effective than tonsillar/buccal administration and without liposomal coating, to generate a significant local immune response both salivary and nasal based on sIgA-anti-GSF [43, 44]. These findings are encouraging; however, the sample size of the study by Childers and collaborators is 21 healthy volunteers. It would be necessary to evaluate the efficacy of active immunization with Streptococcus mutans GSF in a larger group of patients, and to compare the nasal route of administration with the subcutaneous route directly in salivary glands [34, 37, 43, 44].
Among the limitations found in the sample of the present study (20 studies), there is the evaluation of the effect in a short time, since only one article evaluated the effect at 3, 6 and 9 years. Furthermore, the lack of homogeneity in the methodologies, the absence of studies reporting information on CFU/ml/cm2, pH level and salivary buffer capacity, as well as the measurement of the level of salivary sIgA; the lack of a uniform distribution among the experimental branches and the little existence of studies within the Latin American population, do not allow formulating robust conclusions on the efficacy of probiotic systems in the modulation of the oral biofilm and the immune response associated to mucosa to prevent dental caries.
Conclusion
Given the wide methodological heterogeneity of the studies processed in this review, we cannot assure that the use of certain probiotic bacteria constitutes effective systems in the prevention of dental caries, by means of biofilm modulation and mucosal associated immune response. However, based on our analysis we can report:
i. Lactobacillus paracasei was the most prevalent probiotic in the present review, showing promising results that should be evaluated on a larger scale and in different ethnic groups, in future studies.
ii. The regular use of probiotics reduces the level of oral colonization of Streptococcus mutans, but its protective anti-plaque effect would be preserved only for 6 months, after the suspension of the product. We need more evidence on the effect of these probiotic systems on the stimulation of the mucosal associated immune response, and to clarify the scope of this prevention approach.
iii. Probiotics appear to be superior to chemical agents in controlling the cariogenic biofilm, stimulating beneficial commensal species, and increasing salivary buffer capacity.
iv. Certain probiotic strains show comparable benefit to immunotherapy with heterologous antibodies (chicken IgY) in modulating oral biofilm and increasing salivary buffer capacity.
Recommendations
The limited evidence for the importance of probiotics in oral health may be explained by the recent interest, from the scientific community, in this topic. More clinical trials using similar methodology and larger sample sizes are needed to compare different types of probiotic strains and their long-term efficacy. Additionally, new studies should evaluate other variables such as modulation in salivary pH and sIgA levels that play an important role in oral homeostasis. Another recommendation for future double-blind, placebo-controlled studies may be to study the efficacy of using probiotics alone versus probiotics in combination with chicken IgY-based immunotherapy (Ovalgen DC). Indeed, the latter approach has emerged as an innovative product with limited evidence, which has shown encouraging results in preventing dental caries.
Conflicts of Interest
None.
Article Info
Article Type
Review ArticlePublication history
Received: Wed 23, Sep 2020Accepted: Mon 02, Nov 2020
Published: Wed 18, Nov 2020
Copyright
© 2023 Rodríguez ML. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2020.05.03
Author Info
Lesly Damaris Osorio Ayala Juan Fernando Buestán Zambrano Jessica Micaela Yamunaqué Vire María Paz Pinos Gavilanes Rodríguez ML
Corresponding Author
Rodríguez MLSemiology and Diagnostic Clinic, School of Dentistry, University of Cuenca-Ecuador, Cuenca, Ecuador
Figures & Tables
Table 1: Distribution of probiotic species evaluated in terms of efficacy with respect to the control group, in all consensus studies.
|
Bacterial Species |
Nº of studies (%) |
Significant reduction in Sm (%) |
Significant increase in salivary pH/ buffer capacity |
Significant increase in salivary level of sIgA |
Average treatment time in days |
|
L. rhamnosus |
5 (25%) |
3 (60%) |
2 (40%) |
0 |
68,7 days |
|
L. paracasei |
6 (30%) |
5 (83,3%) |
1 (16,6%) |
1 (16,6%) |
128,5 days |
|
L. reuteri |
3 (15%) |
1 (33,3%) |
1 (33,3%) |
0 |
147,6 days |
|
L. acidophilus |
2 (10%) |
2 (100%) |
0 |
0 |
22 days |
|
L. casei |
1 (5%) |
1 (100%) |
0 |
0 |
57 days |
|
L. sporogenes |
1 (5%) |
1 (100%) |
0 |
0 |
30 days |
|
Bacillus coagulans |
2 (10%) |
1 (50%) |
0 |
0 |
10,5 days |
|
Bifidobacterium lactis |
1 (5%) |
1 (100%) |
0 |
0 |
14 days |
|
Streptococcus salivarius |
1 (5%) |
1 (100%) |
0 |
0 |
91,2 days |
References
- Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM et al. (2013) Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J Med Microbiol 62: 875-884. [Crossref]
- Hasslöf P, West CE, Videhult FK, Brandelius C, Stecksén Blicks C (2013) Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res 47: 559-565. [Crossref]
- Dye BA (2017) The Global Burden of Oral Disease: Research and Public Health Significance. J Dent Res 96: 361-363. [Crossref]
- Yadav M, Poornima P, Roshan NM, Prachi N, Veena M et al. (2014) Evaluation of probiotic milk on salivary mutans streptococci count: An in vivo microbiological study. J Clin Pediatr Dent 39: 23-26. [Crossref]
- Rodríguez G, Ruiz B, Faleiros S, Vistoso A, Marró ML et al. (2016) Probiotic compared with standard milk for high-caries children. J Dent Res 95: 402-407. [Crossref]
- Dhamo B, Elezi B, Kragt L, Wolvius EB, Ongkosuwito EM (2018) Does dental caries affect dental development in children and adolescents? Bosn J Basic Med Sci 18: 198-205. [Crossref]
- Abdelwahab DH, Allam GG, Aziz AMA (2018) Effect of xylitol and sugar-free chewing gums on salivary bacterial count of streptococcus mutans and lactobacilli in a group of Egyptian school children of different ages: A randomized clinical trial. Future Dent J 4: 216-220.
- Zaura E, Twetman S (2019) Critical Appraisal of Oral Pre- And Probiotics for Caries Prevention and Care. Caries Res 53: 514-526. [Crossref]
- Kavitha M, Prathima GS, Kayalvizhi G, Sanguida A, Ezhumalai G et al. (2019) Evaluation of Streptococcus mutans serotypes e, f, and k in saliva samples of 6-12-year-old school children before and after a short-term daily intake of the probiotic lozenge. J Indian Soc Pedod Prev Dent 37: 67-74. [Crossref]
- Manmontri C, Nirunsittirat A, Piwat S, Wattanarat O, Pahumunto N et al. (2020) Reduction of Streptococcus mutans by probiotic milk: a multicenter randomized controlled trial. Clin Oral Investig 24: 2363-2374. [Crossref]
- Organización Mundial de la Salud (2019) Reducción de la ingesta de azúcares libres en adultos para reducir el riesgo de enfermedades no transmisibles. Biblioteca Electrónica de Documentación Científica Sobre Medidas Nutricionales (ELENA).
- Pahumunto N, Piwat S, Chanvitan S, Ongwande W, Uraipan S et al. (2020) Fermented milk containing a potential probiotic Lactobacillus rhamnosus SD11 with maltitol reduces Streptococcus mutans: A double-blind, randomized, controlled study. J Dent Sci.
- Jindal G, Pandey RK, Agarwal J, Singh M (2011) A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Eur Arch Paediatr Dent 12: 211-215. [Crossref]
- Koopaie M, Fatahzadeh M, Jahangir S, Bakhtiari R (2019) Comparison of the effect of regular and probiotic cake (Bacillus coagulans) on salivary ph and streptococcus mutans count. Dent Med Probl 56: 33-38. [Crossref]
- Chuang LC, Huang CS, Ou Yang LW, Lin SY (2011) Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Investig 15: 471-476. [Crossref]
- Rungsri P, Akkarachaneeyakorn N, Wongsuwanlert M, Piwat S, Nantarakchaikul P et al. (2017) Effect of fermented milk containing Lactobacillus rhamnosus SD11 on oral microbiota of healthy volunteers: A randomized clinical trial. J Dairy Sci 100: 7780-7787. [Crossref]
- Cildir SK, Sandalli N, Nazli S, Alp F, Caglar E (2012) A novel delivery system of probiotic drop and its effect on dental caries risk factors in cleft lip/palate children. Cleft Palate Craniofac J 49: 369-372. [Crossref]
- Pahumunto N, Piwat S, Chankanka O, Akkarachaneeyakorn N, Rangsitsathian K et al. (2018) Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol Scand 76: 331-337. [Crossref]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097. [Crossref]
- Pinillo León AL, Cañedo Andalia R (2005) El MeSH: Una herramienta clave para la búsqueda de información en la base de datos Medline. Acimed 13.
- Laleman I, Teughels W (2015) Probiotics in the dental practice: a review. Quintessence Int 46: 255-264. [Crossref]
- Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P et al. (2013) The use of probiotic strains in caries prevention: A systematic review. Nutrients 5: 2530-2550. [Crossref]
- Villavicencio J, Villegas LM, Arango MC, Arias S, Triana F (2018) Effects of a food enriched with probiotics on Streptococcus mutans and Lactobacillus spp. salivary counts in preschool children: a cluster randomized trial. J Appl Oral Sci 26: e20170318. [Crossref]
- Teanpaisan R, Piwat S (2014) Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: A randomized placebo-controlled trial. Clin Oral Investig 18: 857-862. [Crossref]
- Badri SM, Felemban EH, Alnajjar GK, Alotaibi FM, Aljahdali ST et al. (2020) Effectiveness of probiotic lozenges and Chlorhexidine mouthwash on plaque index, salivary pH, and Streptococcus mutans count among school children in Makkah, Saudi Arabia. Saudi Dent J.
- Shah SS, Nambiar S, Kamath D, Suman E, Unnikrishnan B et al. (2019) Comparative evaluation of plaque inhibitory and antimicrobial efficacy of probiotic and chlorhexidine oral rinses in orthodontic patients: A randomized clinical trial. Int J Dent 2019: 1964158. [Crossref]
- Kamalaksharappa SK, Rai R, Babaji P, Pradeep MC (2018) Efficacy of probiotic and green tea mouthrinse on salivary pH. J Indian Soc Pedod Prev Dent 36: 279-282. [Crossref]
- Stensson M, Koch G, Coric S, Abrahamsson TR, Jenmalm MC et al. (2014) Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res 48: 111-117. [Crossref]
- Jothika M, Vanajassun PP, Someshwar B (2015) Effectiveness of probiotic, chlorhexidine and fluoride mouthwash against Streptococcus mutans-Randomized, single-blind, in vivo study. J Int Soc Prev Community Dent 5: S44-S48. [Crossref]
- Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B (2009) Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr 49: 349-354. [Crossref]
- Tahmourespour A, Kermanshahi RK (2011) The effect of a probiotic strain (Lactobacillus acidophilus) on the plaque formation of oral streptococci. Bosn J Basic Med Sci 11: 37-40. [Crossref]
- Nishihara T, Suzuki N, Yoneda M, Hirofuji T (2014) Effects of Lactobacillus salivarius-containing tablets on caries risk factors: A randomized open-label clinical trial. BMC Oral Health 14: 110. [Crossref]
- Krüger C, Pearson SK, Kodama Y, Vacca Smith A, Bowen WH et al. (2004) The effects of egg-derived antibodies to glucosyltransferases on dental caries in rats. Caries Res 38: 9-14. [Crossref]
- Shanmugam KT, Masthan KMK, Balachander N, Jimson S, Sarangarajan R (2013) Dental caries vaccine- A possible option? J Clin Diagn Res 7: 1250-1253. [Crossref]
- Nguyen SV, Icatlo FC, Nakano T, Isogai E, Hirose K et al. (2011) Anti-cell-associated glucosyltransferase immunoglobulin Y suppression of salivary mutans streptococci in healthy young adults. J Am Dent Assoc 142: 943-949. [Crossref]
- Hamada S, Horikoshi T, Minami T, Kawabata S, Hiraoka J et al. (1991) Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect Immun 59: 4161-4167. [Crossref]
- Patel M (2020) Dental caries vaccine: are we there yet? Lett Appl Microbiol 70: 2-12. [Crossref]
- Childers NK, Michalek SM, Pritchard DG, McGhee JR (1990) Mucosal and systemic responses to an oral liposome-Streptococcus mutans carbohydrate vaccine in humans. Reg Immunol 3: 289-296. [Crossref]
- Childers NK, Zhang SS, Michalek SM (1994) Oral immunization of humans with dehydrated liposomes containing Streptococcus mutans glucosyltransferase induces salivary immunoglobulin A2 antibody responses. Oral Microbiol Immunol 9: 146-153. [Crossref]
- Smith DJ, Taubman MA (1987) Oral immunization of humans with Streptococcus sobrinus glucosyltransferase. Infect Immun 55: 2562-2569. [Crossref]
- Smith DJ, Taubman MA (1990) Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol 10: 273-281. [Crossref]
- Childers NK, Tong G, Michalek SM (1997) Nasal immunization of humans with dehydrated liposomes containing Streptococcus mutans antigen. Oral Microbiol Immunol 12: 329-335. [Crossref]
- Childers NK, Tong G, Mitchell S, Kirk K, Russell MW et al. (1999) A controlled clinical study of the effect of nasal immunization with a Streptococcus mutans antigen alone or incorporated into liposomes on induction of immune responses. Infect Immun 67: 618-623. [Crossref]
- Childers NK, Tong G, Li F, Dasanayake AP, Kirk K et al. (2002) Humans Immunized with Streptococcus mutans Antigens by Mucosal Routes. J Dent Res 81: 48-52. [Crossref]
