Modelling and Validating Three-Dimensional Human Breast and Cancerous Human Breast Tissues In Vitro
A B S T R A C T
In this study three dimensional (3-D) in vitro models of normal breast and breast cancer tissues were developed to mimic closely the in vivo tissue microenvironment and therefore providing reliable models for in vitro studies as well as testing of novel cancer therapies. Normal and cancerous human breast cell lines were used to construct 3-D artificial tissues, where de-epidermalised dermis (DED) was used as a scaffold for both models. Morphological analyses were conducted using haematoxylin and eosin staining. Biomarkers including keratin 5 and 19 as well as α smooth muscle actin and mucin 1 were used to confirm and validate the reliability of the proposed models using immunohistochemical techniques. Findings suggest that the 3-D in vitro models described in this work can serve as functional models of both human normal and cancerous breast tissues. Multiple structures similar to ducts and lobules of human breast in vivo were observed in 3-D in vitro models by the use of H&E, some breast cancer colonies seen in the cancerous 3-D model were similar to the ducto-lobular structures observed in normal 3-D model of the breast but the former cells were more loosely connected, irregular and largely disorganized. The established 3-D in vitro model of normal breast showed the development of ducto-lobular structures composed of an inner cell layer which was stained positive with α mucin 1 antibody, a biomarker that is characteristic for luminal cells; and also an outer basal layer of cells that was stained positive for α smooth muscle actin, a biomarker of myoepithelial cells.. Keratin staining in 3-D in vitro models also resembled the pattern observed in vivo where keratin 5 was detected in both luminal and myoepithelial cells of normal breast model (NTERT cells), whereas keratin 19 was present in breast cancer model (C2321 cells). These 3-D models successfully recapitulate both normal and pathological tissue architecture of breast tissue and has the potential for various applications in the evaluation of breast cancer progression and treatment.
Keywords
Breast cancer, keratins, alpha smooth muscle actin, mucin 1, three dimensional in vitro models
Introduction
Culturing cells in conventional 2-D conditions is routinely used as an initial model system to evaluate the safety and effectiveness of various potentially therapeutic compounds. Studies that involve culturing cells in flat monolayers, in two dimensional cell cultures often do not reflect the in vivo situation observed in tissues due to the altered geometric and mechanical restrictions enforced on cells [1]. Tissue architecture has been shown to be essential for the maintenance of cell differentiation [2]. In cancer research, the tumour microenvironment and the architecture of extracellular matrix (ECM) are the major factors that determine cancer development and responses to treatment. ECM is a crucial element that promote normal epithelial polarity and differentiation [1]. Moreover, ECM was reported to dictate the phenotype of mammary epithelial cells [3, 4]. Therefore, reliable and robust three-dimensional in vitro tumour models will play an important role in cancer research by providing information on tissue organization and cellular differentiation.
Although current progress in the 3-D culture systems development is very dynamic and advances in tissue engineering have enabled the elaboration of various in vitro models of normal breast and breast cancer, further research is still needed [5]. In particular, difficulties in mimicking breast cancer development and progression in a laboratory setting indicate that there is still a need to develop in vitro human breast models which can more accurately represent breast diseases, such as breast cancers [6].
In this study, we created and described a novel 3-D in vitro models for both normal and cancerous mammary tissues. We have mimicked the ECM characteristics and tissue microenvironment by using ECM components of human DED. By the use of hematoxylin and eosin (H&E) staining, the morphology of normal and cancerous cell arrangements that formed multicellular structures was compared in these 3-D in vitro models. To verify the reliability of 3-D in vitro model of the breast, keratin 5, keratin 19, α-smooth muscle actin (α actin) and MUC 1 biomarkers were used and analysed using immunohistochemical techniques.
Methodology
I Cell Lines
All cell lines were purchased from the American Type Culture Collection (UK). Breast cancer cell lines were grown in RPMI (Gibco Invitrogen, UK) media supplemented with 10% Fetal Bovine Serum – FBS (BioSera, UK), 1% antibiotic (Sigma, UK), supplemented with 1mM of sodium pyruvate (Gibco Invitrogen, UK). Human keratinocytes (NTERT) were grown in Keratinocyte-SFM medium with L-Glutamine, without Calcium Chloride (Gibco Invitrogen, UK) supplemented with Keratinocyte-SFM Supplement Human recombinant Epidermal Growth Factor (2.5µg/L) and Bovine Pituitary Extract (BPE) (25mg/L) (Gibco Invitrogen, UK). All cells were grown at 37ºC in a humidified atmosphere of 5% CO2 with the media being changed regularly depending on the growth rates of different cell lines.
II Three-Dimensional In Vitro Models
NTERT cells were used to create a control model of normal human breast, whereas breast cancer cells MCF7, C2321 and C2335 were used to produce in vitro models of breast cancer. The immortalized human keratinocytes cell line NTERT and the cervical cancer cell lines C33A, C2321 an C2335 were purchased from ATCC (ATCC, UK). All cell lines used were grown on de-epidermalised dermis (DED), immersed in media with the addition of 1 µM estradiol (Sigma, UK). In order to verify the presence of ducto-lobular structures, two antibodies: anti-α actin, that stains myoepithelial cells of the duct and lobule of the breast, and anti-MUC 1, that stains luminal cell in ducts and lobules of the breast, were used. An adjacent normal breast tissue area from breast cancer patient was used as a positive control. Negative controls where primary antibody was omitted and negative isotypic controls were also employed in this study (data not shown).
All cell lines were grown using DED as a scaffold for modelling 3-D in vitro systems. Human skin (Euro Skin Bank, Netherlands) was de-epidermalised using method previously described by Zuk et al. [7]. The de-epidermalised dermis was cut in small sections (1.5 x 1.5 cm,) washed three times in 1% PBS and incubated at 37ºC in a humidified atmosphere of 5% CO2 for 24h with culture media. Cells were then seeded on the top of the de-epidermilised dermis inside a metal ring and after 48 hours, after cell attachment, the ring was removed, and cells were further incubated with media supplemented with 1 µM of estradiol for 3-4 weeks with media replenished regularly. The 3D models were then washed in PBS and placed in 4% paraformaldehyde for 48 hours. After fixation models were washed in PBS, dehydrated in 70%, 90% and absolute ethanol respectively and cleared in xylene before being embedded in paraffin wax.
III Staining
The morphology and characteristics of normal and cancerous cell arrangements in the 3-D in vitro models created were examined by the use of H&E and immunohistochemistry staining techniques. Paraffin-embedded tissue blocks were cut in 6µm thick sections and stained for both H&E or immunohistochemical staining using methods previously described by Zuk et al. [7]. In brief, sections were deparaffinised in xylene and rehydrated through graded alcohols to water. To block endogenous peroxidase sections were incubated in 3% H2O2 in methanol for 10 minutes (Sigma-Aldrich Co. Ltd, Poole, UK). Different epitope retrieval techniques were used, according to (Table 1). To block non-specific binding of immunoglobulin sections were incubated in blocking serum (normal horse serum (Sigma, UK) and PBS in 1:1 proportion) for 10 minutes. Sections were then incubated with primary antibody overnight at 4ºC, and after three PBS washes, they were further incubated with biotinylated secondary antibody for 30 minutes at room temperature. Samples were then rinsed in PBS and then incubated with universal avidin biotin complex (ABC) solution (Vector Laboratories Ltd, UK) for 20 minutes at room temperature. Afterward, samples were washed in PBS and sections were developed using 3,3'-Diaminobenzidine (DAB) (Sigma-Aldrich Co. Ltd, UK) substrate solution for up to 5 minutes. Then samples were briefly rinsed in distilled water and counterstained with Gill’s hematoxylin solution (Sigma-Aldrich Co. Ltd, UK). Sections were differentiated in 1% acid alcohol for a few seconds, washed in running tap water for 5 minutes and dehydrated through 70%, 90% for 2 minutes and two changes of absolute alcohol for 3 minutes each. Finally, samples were cleared in two changes of xylene, and mounted with xylene based mounting medium before being analysed using a light microscope (Nikon eclipse SOi, UK). Images were captured using Image - Pro Express 6.3 program. A list of primary and secondary antibodies is presented in (Table 1) below.
Table 1: Antibodies used for IHC staining and their characteristics.
|
1º Ab |
Ig type |
Concentration |
Antigen retrieval |
Serum |
2 º Ab |
|
Anti α-smooth muscle actin (mouse monoclonal) (Abcam) |
IgG2a
|
2 µg/mL |
0.1% Triton-100, 20 min |
goat |
Goat anti-mouse IgG H&L (Biotin) |
|
Anti-MUC 1 (rabbit monoclonal) (Abcam) |
IgG
|
1.53µg/mL |
0.1% Triton-100, 20 min |
horse |
Biotinylated anti-rabbit IgG |
|
Isotypic control (goat anti-mouse) (Abcam) |
IgG2a |
1.1 µg/mL
|
0.1% Triton-100, 20 min
|
horse |
Biotinylated anti-rabbit IgG |
|
Isotypic control (goat anti-mouse) (Abcam) |
IgG |
1.53 µg/mL |
0.1% Triton-100, 20 min |
horse |
Biotinylated anti-rabbit IgG |
|
Anti-cytokeratin 5 [K5] (rabbit monoclonal) (Abcam) |
IgG |
1 µg/mL |
Citrated buffer x1, heated for 20min |
horse |
Biotinylated anti-rabbit IgG |
|
Anti-cytokeratin 19 [CK19] (mouse monoclonal) (Abcam) |
IgG2a |
1 µg/mL |
Citrated buffer x1, heated for 20min |
horse |
Biotinylated anti-mouse IgG |
Staining of the 3-D In Vitro Models by Confocal Microscopy Analyses
Paraffin-embedded 3-D in vitro models were cut in 6µm sections and prepared as for standard IHC as above. Sections were incubated with primary antibody MUC 1 (Abcam, UK) overnight at 4ºC. On the second day sections were further incubated with secondary antibody - biotinylated anti-rabbit IgG in PBS for 30 minutes at room temperature. Then samples were rinsed in PBS twice for 5 minutes and incubated with avidin biotin complex solution (ABC), (Vectastain, Vector Laboratories, UK) for 20 minutes at room temperature. Samples were further developed with TSA/Fluorescein (PerkinElmer, USA) for 5 minutes and washed in PBS twice for 5 minutes. In the following step sections were washed in PBS and blocked again with goat serum (Sigma, UK) (for α actin) for 10 minutes. Sections were then incubated with primary antibody α actin (Abcam, UK) for 1 hour at room temperature and following PBS washes, sections were further incubated with secondary antibody - Goat anti-mouse IgG H&L (Biotin), for 30 minutes at room temperature. Samples were rinsed in PBS twice for 5 minutes and incubated with avidin biotin complex (ABC) solution for 20 minutes at room temperature. Then samples were developed with TSA/Cy5 (PerkinElmer Life and Analytical Sciences, Inc. Boston, USA) for 5 minutes and washed in PBS twice for 5 minutes. Finally, sections were mounted with DAPI - 4', 6-diamidyno-2-fenyloindol (Sigma, UK). Images of double fluorescent staining were captured using a confocal fluorescent microscope (Leica Microsystems, Wetzlar, Germany), where α actin was stained red and Muc 1 was stained green. Nuclei were counterstained by 6-diamidino-2-phenylindole (blue nuclei).
Results
Multiple structures that resemble duct and lobules of human breast in vivo were observed in the established 3-D in vitro models by the use of H&E and immunohistochemistry staining. Structures were further stained with different keratins in order to reveal and compare the different patterns of keratin staining in normal and cancerous breast tissue. [8-10]. In our models, K5 was detected in both luminal and myoepithelial cells of normal 3-D in vitro model of the breast (Figure 1), whereas K19 was present in 3-D in vitro model of cancerous breast (Figure 2).
Figure 1: K5 staining in 3-D in vitro model of normal human breast; A) K5 was positively stained (arrows) for both luminal and myoepithelial cells; B) H&E of the same area, mag. x 400.
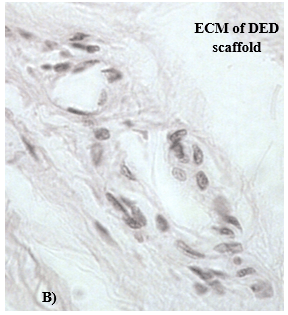
Figure 2: K19 staining in 3-D in vitro model of human breast cancer; A) K19 positive C2321 cells are indicated by arrows; B) H&E of the same section, mag. x 200.
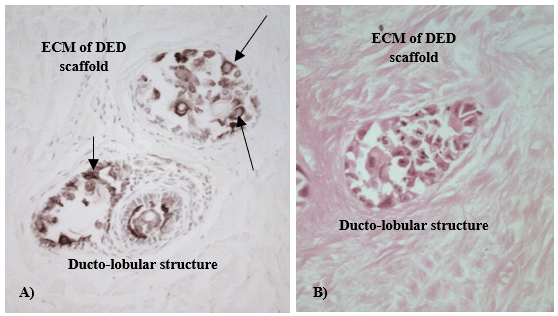
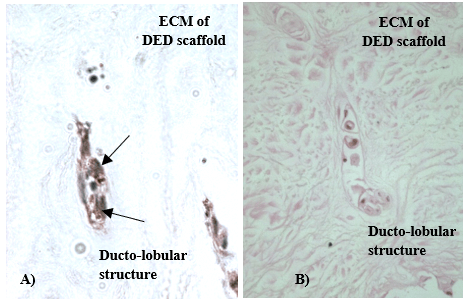
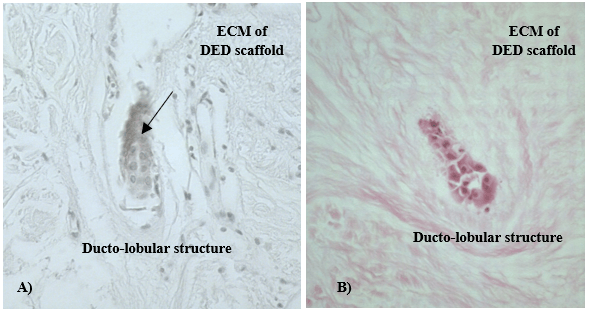
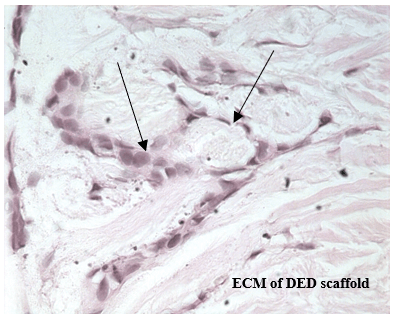
To verify the reliability of 3-D in vitro model of the breast α actin and MUC 1 biomarkers were used in both immunohistochemical and confocal fluorescent microscopy analyses. Biomarker MUC 1 that is normally expressed by luminal cells, was detected inside ducto-lobular structures of 3-D in vitro models and the staining was most intense in the centre of cellular formations (Figure 3). The positive staining of α actin, a biomarker that is normally expressed by myoepithelial cells of the breast, was detected outside ducto-lobular structures formed in 3-D in vitro models (Figure 4). Spatial organization of myoepithelial cells in relation to luminal cells was observed, particularly in 3-D in vitro model of normal breast.
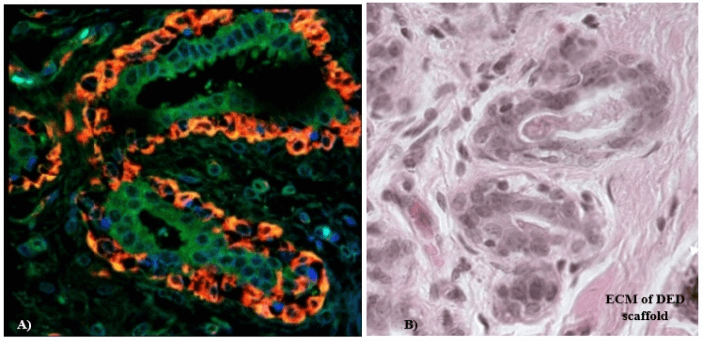
Figure 3: NTERT cells grown in 3-D in vitro model stained with anti MUC1 antibody. A) Cells formed ducto-lobular structures inside the DED scaffold (arrow); B) H&E staining of the same section, mag. x200.
Figure 4: α actin positive staining for NTERT cells grown in 3-D in vitro model. A), B), C) staining was detected outside ducto-lobular structures formed in 3-D in vitro models (arrows), mag x 200; D) H&E of the same section, mag x400.
Figure 5: H&E staining for 3D in vitro cell model established using C2335 cells. Cells were formed colonies similar to the ducto-lobular structures observed in normal 3-D model of the breast, but cancer cells were more loosely connected, irregular and largely disorganized. Cells with different sizes and shapes were visible with large, variably shaped nuclei (arrows); mag. x 400.
Some breast cancer colonies seen in the cancerous 3-D model were similar to the ducto-lobular structures observed in normal 3-D model of the breast, but the former cells were more loosely connected, irregular and largely disorganized (Figure 5).
IHC staining with anti-α-actin and anti-MUC 1 confirmed the presence of ducto-lobular structures in 3-D in vitro model of normal breast. The enhancement of detection was achieved with the use of a double immunofluorescent staining method that was employed for further 3-D model characterization and validation. The ducto-lobular structures found in 3-D in vitro models of breast, were double-stained with anti-MUC 1 antibody (green) and anti–α-actin (red), whereas nuclei of the cells were counter stained using DAPI. Expression of α-actin and MUC 1 in 3-D in vitro model of normal breast (Figure 6) correlated with the staining of control breast tissue section presented in (Figure 7). In breast cancer models established using C2335 cells, cells were also reorganized into structures which are similar to ducto-lobular arrangement observed in 3-D model of normal breast (Figure 8), however, the boundary between the inner and outer layer, was not that clear as seen in NTERT cells.
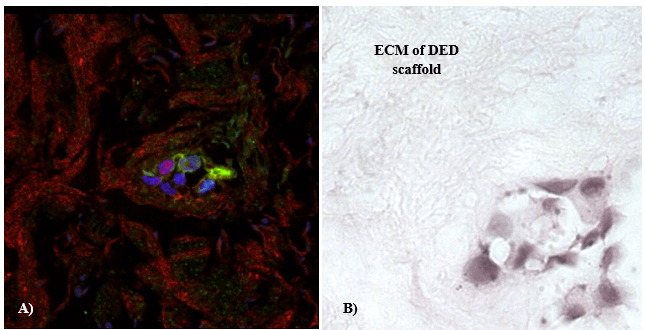
Figure 6: A) Confocal microscopy analyses of NTERT cells grown in 3-D in vitro model, stained with α-actin (red) and MUC 1 (green); white arrows represent α actin, whereas yellow arrows represent MUC 1; mag. x 400; B) H&E staining of the same area.
Figure 7: A) Confocal fluorescent microscopy analyses of the control breast tissue section stained with α-actin (red) and MUC 1 (green); mag. x 400; B) corresponding H&E staining of the same breast tissue sections.
Figure 8: Confocal fluorescent microscopy analyses of α-actin (red) and MUC 1 (green) from 3D in vitro breast cancer model. A) 3-D in vitro model of C2335 breast cancer cells, stained with α-actin (red) and MUC 1 (green); cells were organized in arrangements similar to ducto-lobular structures observed in normal 3-D in vitro model, but the inner and outer layer was not clearly separated; mag. x 400; B) corresponding H&E staining of the same area.
Discussion
New physiologically relevant and cost effective in vitro cellular models are needed to gain a better understanding of the mechanism of human tissue pathology, and to estimate dosage of new therapies, to assess the efficacy, or toxicity of drugs, as well as to evaluate drug penetration into tissue. Current progress in tissue engineering have improved the diversity and quality of 3-D in vitro models which take them one step closer to the in vivo situation. Each of them has some advantages and limitations:
Spontaneous cell aggregation is a technique where the 3-D spheroids can be formed by culturing cells on artificial substrates that stimulate cellular differentiation and maintain cellular function or by spontaneous cell aggregation where spherical cellular conglomerates are generated. Multicellular tumour spheroids are very small structures that are composed with tumour and extensive ECM cells structured in a 3-D arrangement. A number of breast cancer cells and breast cell lines have the ability to spontaneously aggregate during cell culture, to form tissue-like spheroids, such as: human breast carcinoma cell MDA-MB-435, breast carcinoma cell line DU4475 [11, 12]. The multicellular aggregate formation correlates with increased cancer survival and metastasis in vitro and is an important feature of metastatic cancer cells. In the spheroid model proliferating cells are usually present near the surface or on the surface and this is caused by mass transport limitations at greater depths, lack of nutrient penetration and toxic metabolic waste build up [13, 14]. For that reason, this model effectively resembles the situation observed in micrometastases prior to vascularization and intervascular microregions seen in large tumours [5]. The advantage of the spontaneous cell aggregation model is that they are cheap and require little work for preparation. The disadvantage is that spontaneous cell aggregation process can only occur in a small number of cell types, also some cells forms clusters instead of spheroids.
Another technique that enables growing cells as 3-D aggregates or spheroids is a liquid overlay culture. Special conditions need to be created, where adhesive forces between the cells are greater than between the cell and a substrate it is plated on. A spinner flask and gyrators are other methods that prevent cell adherence by inhibiting meaningful contact with the culture vessel wall. Liquid overlay techniques that prevents matrix deposition is the simplest way to achieve such conditions. The advantage of this technique is that many cell lines will undergo spontaneous aggregation. Moreover, even in tumour cell lines that do not aggregate or are difficult to aggregate, spheroid formation can be induced. In this process cells are grown in media over an agar base. Initially cells that are cultured on the agar or reconstituted basement membrane (Matrigel) migrate towards each other and form spheroids. In the following step, cells are growing on the substratum but do not adhere to it and spheroid increases in size [15]. Liquid overlay cultures produce spheroids that maintain the cellular composition and differentiation as seen in tissue in vivo. They can form lobulo-alveolar structures and exhibit differentiated functions such as the capacity to secrete milk.
However, the disadvantage of this model is that adhesion behaviour can be altered as cells have contact with the substratum. The type of media and ECM used are important factors for promoting and supporting cell growth and differentiation. It has been shown that, thick matrices promote differentiation, whereas thin layers of 3-D ECM tumour cells fail to show differentiated phenotypes and form disorganized and apolar cellular masses [16]. It is also important that other media components do not obstruct cell differentiation process. A further limitation is that this method produces only a small number of spheroids [5].
Spinner flask is a common method used for growing cells as a suspension culture in liquid media. This method enables to cultivate greater numbers of spheroids in dynamic suspension when the static environment of liquid overlay cultures is not vital. Spinner flasks are stirred tank bioreactors where mixing maintains the cells in suspension. In this system fluid movement facilitates mass transport of nutrients to, and also wastes from, the spheroids. The disadvantage of this model is that due to high shear forces spheroids architecture is not as well preserved. Also, fragile cells can be damaged by higher fluid turbulence, which affects membrane integrity and cell metabolism. Spinner flask culture developed in the 1970s was the most commonly used method when culturing larger numbers of multicellular spheroids [17]. Roller tubes and gyratory shakers are other methods that are employed for culturing cells in 3-D models.
The gyratory rotation technique is another method that implicates placing cell suspension with media in an Erlenmeyer flask. Erlenmeyer flask is rotated in a gyratory rotation incubator till the time where spheroids of required size are created. In this technique cells have no contact with any substrates [5].
Another technique is a scaffold-based culture system where 3-D in vitro models of the breast can be produced by growing cells on prefabricated scaffolds. In this model cells attach to and migrate along fibres of the scaffold. When cells grow and divide, they manage to fill the scaffold interstices and create 3-D cultures [18]. The properties of obtained cultures can be influenced by the constituents of the scaffold. Collagen and other ECM proteins as well as growth factors are the most frequently used scaffolds. Also, different types of cells such as: established cell lines, primary cells and pieces of tissue can be used in this system [19].
The limitation of this model is its high costs and difficulty when seeding and culturing sensitive cell lines.
In recent times, prefabricated 3-D biodegradable engineered scaffolds represent one of the most promising experimental method for mimicking the structure and properties of tissue in vivo. These scaffolds are composed of synthetic polymers that form 3-D physical support and natural molecules. Models can be used for both: cell culture (in vitro) and tissue regeneration (in vivo). They provide physical and structural support bringing great potential in reproducing not only the structural but also natural physical environment of a living tissue [20]. The biggest limitation of this model is the expense.
Finally, the Rotary Cell Culture System, developed by National Aeronautics and Space Administration (NASA), demonstrates a revolutionary model that simulates microgravity for cells cultured in media, in a dynamic fluid suspension that is mixed by minimal hydrodynamic forces. In this system, the culture flask rotates whole on its horizontal axis and provides end over end mixing of the cells. Shear forces and fluid turbulences are minimized as the flask (vessel) is entirely filled with media. Aeration is facilitated through a semi permeable membrane that eliminates bubbles, which are normally responsible for hydrodynamic forces production. Reduced turbulence affected the physical properties of a culture, like production of spheroids larger in size, considerably more differentiated morphologically and phenotypically than in the ones cultured in spinner flasks. In this model multiple cell types can be co-cultured. The biggest limitation of this model is its expense [5].
Each of the represented models has its advantages and restrictions. The 3-D in vitro model proposed in this study is an inexpensive alternative to commercially available systems, where the 3-D architecture of tissue was recreated and maintained successfully. In our model cells are surrounded by a natural environment of human dermis, where nutrients are naturally transported from media. Therefore, there is no mass transport limitations at greater depths, or problem with lack of nutrient penetration and toxic metabolic waste build up that are often seen in other commercially available 3-D in vitro models. DED scaffold used in our model provides natural physical and structural support creating in vivo like environment of a living tissue. This promising technique has offered a low-cost solution to culture a variety of cell types in a well-defined environment under 3-D culture conditions.
The biology of normal luminal and myoepithelial cells is the key to understand breast cancers. Terminal ducto-lobular unit in the breast is the structure from which breast cancers predominantly arise. This structure is made up of two types of epithelial cells; the inner layer of luminal cells that could be milk secreting cells and an outer basal layer composed of contractile myoepithelial cells. The two layers of cells are separated from the interstitial stroma by an intact basement membrane [21, 22]. The glandular luminal epithelial cells are polarized with specific basolateral and apical membrane. Luminal cells that line the apical surface of the normal breast duct have secretory properties. The myoepithelial cells that have both contractile muscle and epithelial properties play a part in the formation of basement membrane. For the reason that carcinoma of the breast arises mainly in the luminal epithelial compartment, not much attention was paid in the past to the role of the surrounding cells in mammary gland [22-24]. The fact that intact myoepithelial cells are significant factors of normal breast development and differentiation is well known now and myoepithelial cells are gaining more attention in cancer research [22-24].
Several myoepithelial proteins demonstrated the ability to inhibit epithelial tumour formation. Examples of proteins that have tumour suppressive activities are: α-actin, K5, α 6 integrin, connexin 43, myoepithelium-derived serine proteinase inhibitor (MEPI), relaxin, maspin, and activin [22, 25-31]. Paracrine interactions between luminal and myoepithelial cells are known to be important for establishing epithelial cell polarity, regulation of cell cycle progression, and inhibition of cell migration and invasion. Better understanding of myoepithelial cell function and their role in tumour progression may lead to their exploitation for cancer therapy and prevention [32].
3-D in vitro systems have been proven to be important experimental tools for cancer research. Serving as low-cost screening platforms they are important models to study the mechanisms of tumour growth, its progression, tumour cell loss of differentiation and the response to therapeutics. When cells are cultured in 2-D environment they lose important signals from ECM, which plays fundamental function in normal homeostasis [33]. Growing cells in 3-D conditions enable restoring some of the important signals. In this study culturing normal and cancer cells in 3-D model of the breast allowed phenotypic discrimination between nonmalignant NTERT cells that formed polarized ducto-lobular structures and malignant proliferating mammary cells that were mainly disorganized and nonpolar. Some breast cancer colonies seen in the cancerous 3-D model were similar to the ducto-lobular structures observed in normal 3-D model of the breast, but the former cell were more loosely connected, irregular and largely disorganized. All cell phenotypes could be distinguished in the 3-D in vitro model of the breast cancer, such as non-proliferating, proliferating and necrotic cells, similar to the situation observed in tumours in vivo. Interactions between DED and epithelial cells enabled development of both normal and neoplastic breast 3-D in vitro models.
The 3-D in vitro systems proposed in this study can serve as reliable in vitro models of normal breast and breast cancer. Standard IHC staining and confocal microscopy analyses confirmed the presence of ducto-lobular structures in the model of normal breast. In this study we compared the expression of keratins, the major structural proteins of epithelial cells, in both normal and cancerous 3-D in vitro models. Keratins are divided into two groups of acidic type I (K9-K20) and basic type II (K1-K8) keratins and its main function is to maintain the epithelial cell integrity of cytoskeleton [34-36]. They also play important role in cell signalling, stress responses and apoptosis [35, 37]. Keratins have a defined distribution pattern in epithelial tissues [38, 39]. Cells lining the lumen of normal breast ducts express keratins: 8, 18, 19 and 7 whereas the basal cell layer of the mammary duct express keratins: 5/14, 5/6 or 17. Myoepithelial cells will express: K5, K14, K17, smooth muscle actin, calponin, p63 [40] (Table 2).
Similarly, breast-associated adenocarcinomas express the same markers as the normal mammary gland. One of the main features of transition from normal to cancerous state is the alteration in the cytoskeletal structure [41]. Breast cancers express either luminal or basal keratins. Breast carcinoma originates from luminally differentiated epithelial cells where 85% carcinomas are ductal (luminal type and basal type) and 15% lobular. Both lobular and ductal carcinomas arise in the terminal ducto-lobular unit [40]. Dissimilarity in keratins expression are often used to distinguish lobular from ductal carcinomas. K5 and 10 are commonly expressed by lobular carcinomas. In contrast, they are absent or expressed at low levels in most ductal carcinomas in situ (DCIS) [40]. In this study the expression of K5 and 19 was studied. Table 2 represents different pattern of keratin expression in non-malignant and cancerous breast tissue.
Table 2: Keratins expression in non-cancerous breast tissue, where “-” represents negative staining, “+” represents weak positive staining, “++” represents positive staining and “+++” represents strongly positive staining.
|
|
Normal tissue |
Malignant tissue |
|||
|
Neutral-basic or acidic |
Acidic |
Neutral-Basic |
Acidic |
Neutral-Basic |
|
|
Type of keratin |
19 |
5 |
19 |
5 |
|
|
Breast Complex epithelia |
Basal and myoepithelial cells |
++ |
+++ |
++ |
- |
|
Luminal cells |
+++ |
+ |
++ |
- |
|
Multiple structures that resemble duct and lobules of human breast in vivo were observed in the established 3-D in vitro models. Structures were further stained with different keratins in order to reveal and compare the different patterns of keratin staining in normal and cancerous breast tissue. Normal mammary epithelial cells express mainly K5 whereas breast cancer cells produce K19 [8-10]. In our models K5 was detected in both luminal and myoepithelial cells of normal 3-D in vitro model of the breast (Figure 1), whereas K19 was present in 3-D in vitro model of cancerous breast tissue (Figure 2). One of the features of cancer cell is that it losses the specific differentiated appearance of normal cell over the time. The nuclei of malignant cells become large, which indicates the increased synthesis of DNA [33]. Malignant cells are also less rigorously regulated by cell-cell and cell-matrix interactions in comparison to normal cells. They are less adhesive than normal cells due to reduced expression of cell surface adhesion molecules. Cancer cells are less firmly attached to each other or to normal cells, they also poorly adhere to the ECM. They are rounder than normal cells and can easily metastasize and invade surrounding tissue [5, 42]. Morphological changes between normal and cancer cells cultured in 3-D in vitro models include alteration in the nuclear envelope. Breast cancer cells have enlarged nuclei (Figure 3) in comparison to the normal cells (Figure 1). Increase in nuclear volume is usually from the increased chromosome content and increased DNA synthesis [33].
The ducto-lobular structures formed in the normal breast tissue model were composed of two types of epithelial cells, the inner cell layer that show luminal cells characteristic (positively stained with MUC 1 biomarker), and an outer basal layer that exhibit myoepithelial cells characteristics (positively stained with α actin biomarker). Similar structures were observed in breast cancer model but staining with α actin and MUC 1 show only uncompleted differentiation. These disturbed cells organization are commonly seen in breast cancers, where cancerous mammary cells have an impaired ability to differentiate to myoepithelial and alveolar cells [43]. The phenotype of breast cancer cells, their reaction to proliferation and differentiation stimuli, as well as mammary cells polarity is also altered [44-46]. Moreover, many breast cancers lack myoepithelial cells and even if the myoepithelial cells are detected they are often loosely connected and irregular, whereas luminal cells are largely disorganized [47-51]. The cell-cell interactions and complex 3-D network of cell–matrix successfully formed in 3-D in vitro models presented in this study is of a great value. 3-D models successfully recapitulate both normal and pathological tissue architecture and offer physiologically relevant systems to study the disease in breast tissue.
Acknowledgements
This work was supported by the Middlesex University, particularly in the award of a Postgraduate Research Studentship that provided the necessary financial support for this research.
Funding
This work is funded by the Middlesex University Postgraduate Research Studentship.
Conflicts of Interest
None.
Abbreviations
α-actin: alpha smooth muscle actin
DED: de-epidermalised dermis
ECM: extracellular matrix
K: keratin
Article Info
Article Type
Research ArticlePublication history
Received: Thu 02, Apr 2020Accepted: Thu 16, Apr 2020
Published: Tue 21, Apr 2020
Copyright
© 2023 Anna Karolina Zuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.05
Author Info
Anna Karolina Zuk Beata Burczynska Dong Li Lucy Ghali Stephen Dilworth Xuesong Wen
Corresponding Author
Anna Karolina ZukDepartment of Natural Sciences, Middlesex University, The Burroughs, Hendon, London, UK
Figures & Tables
Table 1: Antibodies used for IHC staining and their characteristics.
|
1º Ab |
Ig type |
Concentration |
Antigen retrieval |
Serum |
2 º Ab |
|
Anti α-smooth muscle actin (mouse monoclonal) (Abcam) |
IgG2a
|
2 µg/mL |
0.1% Triton-100, 20 min |
goat |
Goat anti-mouse IgG H&L (Biotin) |
|
Anti-MUC 1 (rabbit monoclonal) (Abcam) |
IgG
|
1.53µg/mL |
0.1% Triton-100, 20 min |
horse |
Biotinylated anti-rabbit IgG |
|
Isotypic control (goat anti-mouse) (Abcam) |
IgG2a |
1.1 µg/mL
|
0.1% Triton-100, 20 min
|
horse |
Biotinylated anti-rabbit IgG |
|
Isotypic control (goat anti-mouse) (Abcam) |
IgG |
1.53 µg/mL |
0.1% Triton-100, 20 min |
horse |
Biotinylated anti-rabbit IgG |
|
Anti-cytokeratin 5 [K5] (rabbit monoclonal) (Abcam) |
IgG |
1 µg/mL |
Citrated buffer x1, heated for 20min |
horse |
Biotinylated anti-rabbit IgG |
|
Anti-cytokeratin 19 [CK19] (mouse monoclonal) (Abcam) |
IgG2a |
1 µg/mL |
Citrated buffer x1, heated for 20min |
horse |
Biotinylated anti-mouse IgG |
Table 2: Keratins expression in non-cancerous breast tissue, where “-” represents negative staining, “+” represents weak positive staining, “++” represents positive staining and “+++” represents strongly positive staining.
|
|
Normal tissue |
Malignant tissue |
|||
|
Neutral-basic or acidic |
Acidic |
Neutral-Basic |
Acidic |
Neutral-Basic |
|
|
Type of keratin |
19 |
5 |
19 |
5 |
|
|
Breast Complex epithelia |
Basal and myoepithelial cells |
++ |
+++ |
++ |
- |
|
Luminal cells |
+++ |
+ |
++ |
- |
|

References
- Roskelley CD, Bissell MJ (1995) Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem Cell Biol 73: 391-397. [Crossref]
- Vidi PA, Bissell MJ, Lelièvre SA (2013) Three-Dimensional Culture of Human Breast Epithelial Cells: The How and the Why. Methods Mol Biol 945: 193-219. [Crossref]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P et al. (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137: 231-245. [Crossref]
- Sun T, Jackson S, Haycock JW, MacNeil S (2006) Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 122: 372-381. [Crossref]
- Kim JB, Stein R, O'Hare MJ (2004 ) Three-dimensional in vitro tissue culture models of breast cancer-- a review. Breast Cancer Res Treat 85: 281-291. [Crossref]
- Holliday DL (2010) A three-dimensional in vitro model of breast cancer: Toward replacing the need for animal experiments. Altern Lab Anim 38 Suppl 1: 41-44. [Crossref]
- Karolina Zuk A, Wen X, Dilworth S, Li D, Ghali L (2017) Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J Biomed Res 31: 240-247. [Crossref]
- Kabir NN, Rönnstrand L, Kazi JU (2017) Keratin 19 expression correlates with poor prognosis in breast cancer. Mol Biol Rep 41: 7729-7735. [Crossref]
- Choi I, Gudas LJ, Katzenellenbogen BS (2000) Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region. Mol Cell Endocrinol 164: 225-237. [Crossref]
- Trask DK, Band V, Zajchowski DA, Yaswen P, Suh T et al. (2000) Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci U S A 87: 2319-2323. [Crossref]
- Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP (2000) Effects of Thomsen-Friedenreich antigen-specific peptide P-30 on beta-galactoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res 60: 2584-2588. [Crossref]
- Langlois AJ, Holder WD Jr, Iglehart JD, Nelson Rees WA, Wells SA Jr et al. (1979) Morphological and biochemical properties of a new human breast cancer cell line. Cancer Res 39: 2604-2613. [Crossref]
- Glinsky GV, Glinsky VV (1996) Apoptosis amd metastasis: a superior resistance of metastatic cancer cells to programmed cell death. Cancer Lett 101: 43-51. [Crossref]
- Fidler IJ (1973) The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9: 223-227. [Crossref]
- Yuhas JM, Li AP, Martinez AO, Ladman AJ (1977 ) A Simplified Method for Production and Growth of Multicellular Tumor Spheroids A Simplified Method for Production and Growth of Multicellular Tumor Spheroids. Cancer Res 37: 3639-3643. [Crossref]
- Wolff MS, Collman GW, Barrett JC, Huff J (1996) Breast cancer and environmental risk factors: epidemiological and experimental findings. Annu Rev Pharmacol Toxicol 36: 573-596. [Crossref]
- Sutherland RM, Inch WR, McCredie JA, Kruuv J (1970) A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med 18: 491-495. [Crossref]
- Bell E (1995) Strategy for the selection of scaffolds for tissue engineering. Tissue Eng 1: 163-179. [Crossref]
- Jacquot J, Spilmont C, Burlet H, Fuchey C, Buisson AC et al. (1994) Glandular-like morphogenesis and secretory activity of human tracheal gland cells in a three-dimensional collagen gel matrix. J Cell Physiol 161: 407-418. [Crossref]
- Tan W, Krishnaraj R, Desai TA (2001) Evaluation of nanostructured composite collagen--chitosan matrices for tissue engineering. Tissue Eng 7: 203-210. [Crossref]
- Jones C, Mackay A, Grigoriadis A, Cossu A, Reis Filho JS (2004) Expression Profiling of Purified Normal Human Luminal and Myoepithelial Breast Cells : Identification of Novel Prognostic Markers for Breast Cancer. Cancer Res 64: 3037-3045. [Crossref]
- Gudjonsson T, Rønnov Jessen L, Villadsen R, Rank F, Bissell MJ (2002) Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115: 39-50. [Crossref]
- Slade MJ, Coope RC, Gomm JJ, Coombes RC (1999) The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp Cell Res 247: 267-278. [Crossref]
- Péchoux C, Gudjonsson T, Ronnov Jessen L, Bissell MJ, Petersen OW (1999) Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol 99: 88-99. [Crossref]
- Tobacman JK, Tobacmani K (1997) Filament Disassembly and Loss of Mammary Myoepithelial Cells after Exposure to λ -Carrageenan Filament Disassembly. 2823-2826.
- Zajchowski DA, Band V, Trask DK, Kling D, Connolly JL (1990 ) Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci U S A 87: 2314-2318. [Crossref]
- Sager R, Anisowicz A, Neveu M, Liang P, Sotiropoulou G (1993) Identification a candidate by differential tumor display as suppressor. FASEB J 7: 964-970. [Crossref]
- Hirschi KK, Xu CE, Tsukamoto T, Sager R (1996) Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ 7: 861-870. [Crossref]
- Xiao G, Liu YE, Gentz R, Sang QA, Ni J et al. (1999) Suppression of breast cancer growth and metastasis by a serpin myoepithelium-derived serine proteinase inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci U S A 96: 3700-3705. [Crossref]
- Bani D, Riva A, Bigazzi M, Bani Sacchi T (1994) Differentiation of breast cancer cells in vitro is promoted by the concurrent influence of myoepithelial cells and relaxin. Br J Cancer 70: 900-904. [Crossref]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M et al. (1994) Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263: 526-529. [Crossref]
- Polyak K, Hu M (2005) Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia 10: 231-247. [Crossref]
- GMW Margaret Barton Burke (2010) “Cancer Therapies” 11-16.
- Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63: 345-382. [Crossref]
- Alix Panabières C, Vendrell JP, Slijper M, Pellé O, Barbotte E et al. (2009) Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res 11: R39. [Crossref]
- Sheng S, Barnett DH, Katzenellenbogen BS (2008) Differential estradiol and selective estrogen receptor modulator (SERM) regulation of Keratin 13 gene expression and its underlying mechanism in breast cancer cells. Mol Cell Endocrinol 296: 1-9. [Crossref]
- Coulombe PA, Omary MB (2002) ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14: 110-122. [Crossref]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31: 11-24. [Crossref]
- Moll R, Schiller DL, Franke WW (1990) Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol 111: 567-580. [Crossref]
- Yeh IT, Mies C (2008) Application of immunohistochemistry to breast lesions. Arch Pathol Lab Med 132: 349-358. [Crossref]
- Jing Y, Zhang J, Waxman S, Mira y Lopez R (1996) Upregulation of cytokeratins 8 and 18 in human breast cancer T47D cells is retinoid specific and retinoic acid receptor dependent. Differentiation 60: 109-117. [Crossref]
- Cooper GM (2000) The Development and Causes of Cancer. The cell, A Molecular Approach. 2nd edition.
- Rudland PS (1993 Epithelial stem cells and their possible role in the development of the normal and diseased human breast. Histol Histopathol 8: 385-404. [Crossref]
- Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M et al. (1996) The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol 132: 1115-1132. [Crossref]
- Birchmeier W, Behrens J (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1198: 11-26. [Crossref]
- Fish EM, Molitoris BA (1994) Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med 330: 1580-1588. [Crossref]
- Li P, Barraclough R, Fernig DG, Smith JA, Rudland PS (1998) Stem cells in breast epithelia. Int J Exp Pathol 79: 193-206. [Crossref]
- Nagle RB, Böcker W, Davis JR, Heid HW, Kaufmann M et al. (1986) Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 34: 869-881. [Crossref]
- Wetzels RH, Holland R, van Haelst UJ, Lane EB, Leigh IM et al. (1989) Detection of basement membrane components and basal cell keratin 14 in noninvasive and invasive carcinomas of the breast. Am J Pathol 134: 571-579. [Crossref]
- Malzahn K, Mitze M, Thoenes M, Moll R (1998) Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch 433: 119-129. [Crossref]
- Polyak K (2007) Breast cancer : origins and evolution. J Clin Invest 117: 3155-3163. [Crossref]
