MMP-9 Shedding Releases Truncated CD73 from Astrocytes
A B S T R A C T
Previously we had reported that astrocytes physiologically express high levels of CD73 in their membrane, converting extracellular AMP to immune suppressive adenosine, mediates an anti-inflammatory effect. Following an interaction with effector T cells (CD4+CD25-), astrocytes lost most of their membrane expressed CD73, which rendered astrocytes’ immune suppressive function and accelerated neural inflammation such as EAE. Here, we investigated the mechanism leading to the loss of membrane CD73 in astrocytes. Our results revealed that there was no significant difference in Cd73 mRNA expressions between CD73high and CD73low astrocytes. Membrane shedding of CD73 by matrix metalloproteinase-9 (MMP-9) accounted for its membrane loss in astrocytes; meanwhile, C terminal truncated CD73 could be found in the medium of induced CD73low astrocytes. With an MMP-9 inhibitor in existence, the shedding of CD73 in wt-astrocytes, when interacted with CD73-/- effector CD4 cells, was almost completely blocked, and the production of pro-inflammatory cytokines, such as IL-17 and IFNγ, from interacted CD73-/- effectors, were significantly decreased. However, when a CD73 inhibitor was added together with MMP-9 inhibitor, decreased production of pro-inflammatory cytokines were completely restored. As conclusion, our findings suggested that under active inflammatory condition, MMP-9 releases CD73 from astrocytes. The block of CD73 shedding in astrocytes by the addition of MMP-9 inhibitor could significantly decrease the activation of interacted effector T cells.
Keywords
Astrocytes, CD73, MMP-9, shedding
Introduction
Ecto-5’-nucleotidase (CD73) is a critical enzyme involved in the modulation of purinergic signaling and participates in immune regulation by controlling extracellular adenosine production [1-4]. Physiologically, CD73 located on the surface of some immune regulatory cells with high amount, including regulatory T (Treg) cells, dendritic cells and T cell receptor (TCR)γδ T cells; which help to create an environment with relatively high adenosine concentration for maintaining immune homeostasis [5-7]. Under pathological conditions, such as stress and inflammation, CD73-positive cells may lose most of their surface located CD73 or even change to CD73-negative cells. This procedure hindered the immune-suppressive effect of adenosine by decreasing its local production and was considered as a pro-inflammatory transformation.
Multiple sclerosis (MS) is the prototypical inflammatory demyelinating disease of the central nervous system (CNS). Experimental autoimmune encephalomyelitis (EAE) is the most commonly used experimental model for the human inflammatory demyelinating disease, multiple sclerosis [8]. Previously we had reported that CD73 is highly expressed on the surface of normal astrocytes. However, when local inflammation was existing, astrocytes lost most of their surface CD73, leading to significantly inhibited local adenosine production, hence hindering its immune suppressive effect and promoted EAE [9]. As a continuous work, we extended our investigation to the mechanism inducing the loss of membrane CD73 in astrocytes, and we believe the clarification of this mechanism could help us understand more of local immune disorders as well as find a potential therapeutic target. This may also benefit the study of other fields, since CD73 is not only a critical regulator in autoimmune diseases but also has a great impact in oncology [10-17].
Materials and Methods
I Materials
Female C57BL/6 (B6) and ARA1-/- and CD73-/- mice on a B6 background (all 12 to 14 weeks old) were purchased from the National Resource Center for Mutant Mice of China (NRCMM) (Nanjing, Jiangsu, China). The mice were housed and maintained in the animal facilities of Tianjin Medical University. Institutional approval was obtained, and institutional guidelines regarding animal experimentation were followed. Phycoerythrin (PE)-labeled anti-mouse GLAST and fluorescein isothiocyanate (FITC)-labeled anti-mouse CD73 were purchased from eBioscience (ThermoFisher Scientific, Beijing, China). Biotrak MMP-9 Activity Assay system, the product of GE Healthcare (Code: RPN2634), selective MMP-9 inhibitor, MMP-9 Inhibitor II (Sigma-Aldrich, 444293) and selective MMP-2 inhibitor, MMP-2 Inhibitor III-CAS 704888-90-4 (Sigma-Aldrich, 444288) were purchased from Sigma (Sigma-Aldrich, USA).
II The Induction of EAE
EAE was induced in either wild type C57BL/6 (B6) mice or CD73-/- mice with B6 genetic background. All the mice were 12 weeks old female. EAE was induced by active induction following a published method [18]. Briefly, the mice were injected (IP) with 150 ng pertussis toxin. Two hours before they were immunized, the mice were given subcutaneous injections of 200 μl of 150 μg myelin oligodendrocyte glycoprotein (MOG35-55) emulsified in complete Freund’s adjuvant (CFA) (Sigma, St. Louis, MO) in 6 locations on the flank and one location at the tail base. The mice were monitored every other day for the development of clinical symptoms. Typical EAE symptoms usually appeared 12-14 days after immunization.
III The Interaction of Isolated Astrocytes and CD4 T Cells
A single-cell suspension was prepared from the brains of naïve wild type or CD73-/- mice. Astrocytes were gathered by autoMACS (Miltenyi Biotec, Germany) isolation. Briefly, cells were first incubated with biotin-conjugated anti-mouse GLAST (ACSA-1) antibody, and were then incubated with magnetic microbeads conjugated anti-biotin antibody. GLAST+ cells were sorted by positive selection on an autoMACS separator column to obtain astrocytes. Isolated astrocytes were cultured in DMEM/F12 medium supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator. When cells reached 80% confluence, usually 12-14 days after culture, astrocytes were primed by the treatment of LPS (50 ng/ml) plus IFN-γ (10 ng/ml) for 1 day.
Meanwhile, by two-steps autoMACS isolation CD73-/- CD4+CD25- effector T cells were isolated from spleens of EAE induced CD73-/- mice. A negative selection kit for CD4 cells (Miltenyi Biotec, Germany) was used firstly, followed by a CD25+ cell depletion procedure. Splenocytes were incubated for 20 min at 4°C in a cocktail of biotin-conjugated antibodies to label all non-CD4 cells and were then incubated with streptavidin-conjugated magnetic microbeads (Miltenyi Biotec, Germany) for 15 min at 4°C. Magnetic microbeads labeled and unlabeled cells were separated on an autoMACS separator column, and the unlabeled cells were harvested and considered as CD4+ cells. CD4+ cells were further incubated with a FITC-conjugated anti-mouse CD25 antibody and anti-FITC antibody-conjugated magnetic microbeads, then CD25+ cells were removed by running a depletion protocol on an autoMACS separator. For creating the interaction, LPS/IFN-γ primed astrocytes were thoroughly washed with DMEM/F12 medium, incubated with 10 μg/ml of MOG35-55 or non-target antigen (NT-Ag) for 30 min and thoroughly washed. Approximately 5×105 purified CD4 cells were added to each well of astrocytes in a 24-well cell culture plate. Two days later, cell culture suspensions were discarded, bottom attached cells were washed, treated with EDTA and harvested. CD73 in GLAST+ cells were analysed by flow cytometry.
IV The Detection of Cd73 mRNA Expression in Astrocytes
After the two days interaction of wt-astrocytes and CD73-/- effectors, cell culture suspensions were discarded; bottom attached cells were thoroughly washed with PBS and soaked in 500 μl TRIZOL reagent for 30 mins. Total RNA was extracted from these TRIZOL treated cells, and wild type Cd73 mRNA was quantitated by RT-qPCR with specially designed primers selectively amplifying wild type Cd73 cDNA from wt-astrocytes but not from CD73-/- CD4 cells. Ct value of each sample was calibrated to the average cell number of astrocytes of each group, while the average cell number was determined by flow cytometry analysis in parallel wells. Their ratio to no antigen control group was represented as relative Cd73 transcript expression in each astrocytes group.
V Immunoprecipitation of CD73
For immunoprecipitation of CD73 from the culture medium of interacted astrocytes and CD4 effectors, the collected medium was incubated with anti-CD73 antibodies at 4 ℃ for 6 hrs under gentle agitation; then protein A/G agarose beads were added and incubated at 4 ℃ for another 4 hrs under agitation. The mixture was centrifuged and washed with PBS. The pellet was suspended in SDS-PAGE loading buffer, subjected to SDS-PAGE and western blot assay. Following SDS-PAGE, some samples were transferred to PVDF membrane and visualized by coomassie blue R-250 staining. The band corresponding to the location of CD73 was sent to C terminal amino acid sequencing.
VI The Detection of Active Form of MMP-9
Extracellular MMP-9 activity was evaluated by a Biotrak Activity system (GE Healthcare, RPN2634) following the manufacturer’s protocol. Medium samples, collected from interacted astrocytes and CD4 cells, were diluted with assay buffer and tested in triplicate. Briefly, 100 μl of diluted sample was added to anti-MMP-9 antibody-coated 96 well microplate and kept at 4°C overnight. It was washed thoroughly and 100 μl of enzyme solution added, containing the pro form of an inactive detection enzyme which can only be activated by bottom captured active MMP-9. After an incubation at 37°C for 2 hrs, the activity of the detection enzyme was measured using a specific chromogenic peptide substrate; the resultant colour is read at 405 nm. The amount of active MMP-9 in each sample was determined by interpolation from a standard curve.
Results
I The Interaction with Effector CD4 Cells Decreased Membrane CD73 But Not Cd73 mRNA Expression in Astrocytes
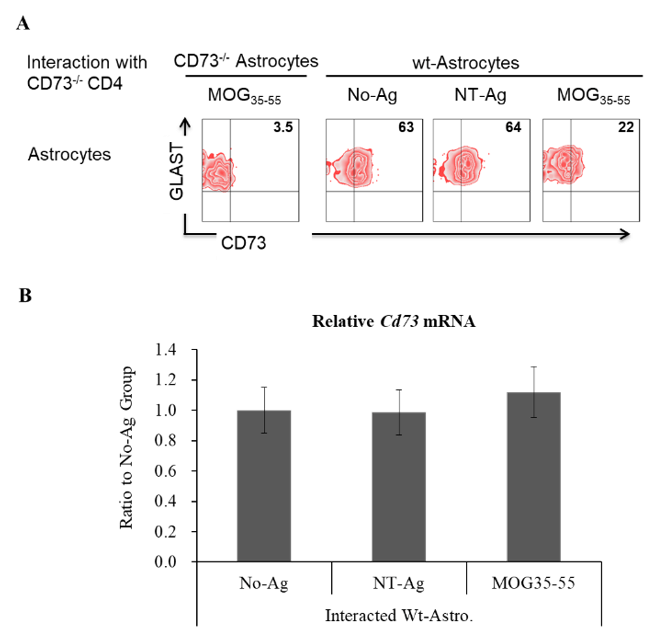
After the interaction with EAE-effectors membrane, CD73 in astrocytes was evaluated by flow cytometry, while Cd73 mRNA expression was determined by qPCR. Figure 1A showed that only with the addition of MOG35-55 but not NT-Ag, the interacted astrocytes lost most of their surface located CD73. However, as far as Cd73 mRNA expression was concerned, there was no significant difference among different groups of astrocytes.
Figure 1: Membrane CD73 and CD73 mRNA expression in differently treated astrocytes. Astrocytes were isolated from naïve B6 mice of wild type and CD73-/-, in vitro cultured and primed with LPS (50 ng/ml) plus IFN-γ (10 ng/ml) for 1 day. Primed astrocytes were pulsed with or without certain antigens, 10 μg/ml of MOG35-55, non-target antigen (NT-Ag) or vehicle, then subjected to the interaction with CD73-/- effector CD4 cells isolated from EAE induced CD73-/- mice. Two days later, membrane CD73 in astrocytes was determined by western blot (A), while Cd73 mRNA was detected by qPCR (B). A) A representative western blotting result. B) Cd73 mRNA expression in different astrocytes (n=6).
II C-terminal Truncated CD73 was Released into Medium
If significantly decreased surface CD73 in astrocytes was due to CD73 being catalyzed and released from astrocytes’ membrane, CD73 was expected to be immunoprecipitated from the culture medium in which MOG35-55 specifically interacted astrocytes and CD73-/- CD4 effectors were cultured. Figure 2A confirmed that CD73 could be immunoprecipitated as expectation, only from the medium of MOG35-55 specifically interacted astrocytes and CD73-/- CD4 effectors. When compared with CD73 in cell membrane extract medium, immunoprecipitated CD73 (MI-CD73) showed a smaller molecular size than membrane extracted CD73 (ME-CD73), shown in (Figure 2B). Further C-terminal amino acid sequencing revealed that MI-CD73 is C-terminal truncated.
Figure 2: C-terminal truncated CD73 was released into medium. CD73 was immunoprecipitated from the medium of interacted astrocytes and CD4 effectors, analysed by western blot and C-terminal amino acid sequencing. A) Western blot result of immunoprecipitations from the medium of interacted astrocytes and CD4 effectors. B) Comparison of CD73 in membrane extract and medium immunoprecipitation. C) C-terminal amino acid sequencing result of immunoprecipitated CD73.
III MMP-9 Catalyzed CD73 from Interacted Astrocytes
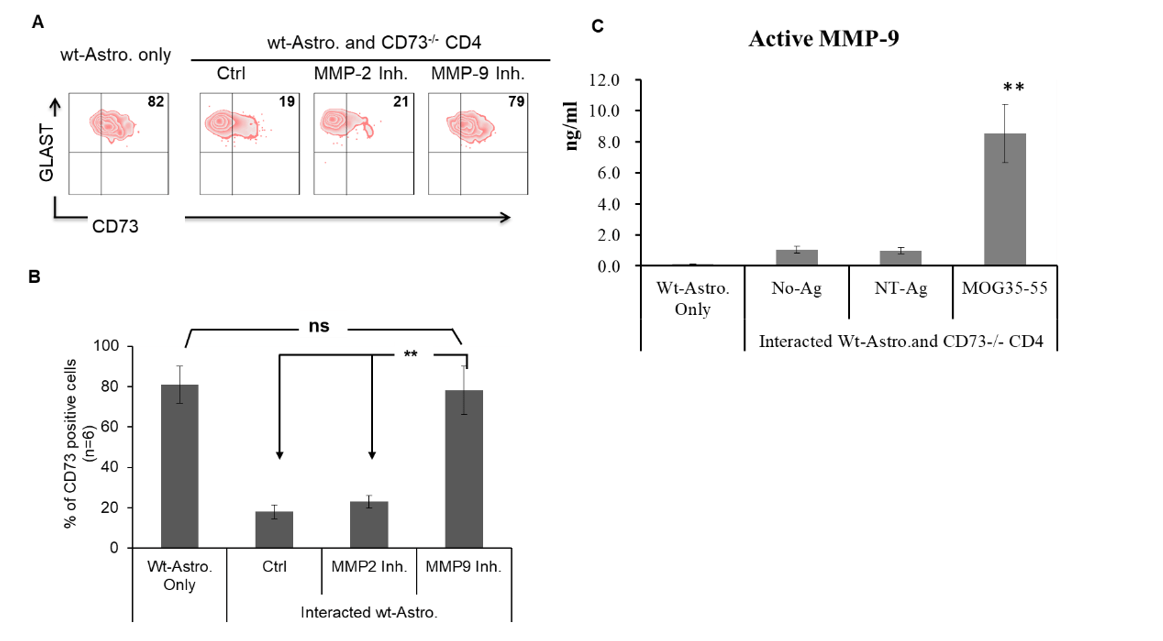
To verify whether it is a certain type of matrix metalloproteinase (MMP) catalyzes CD73 from astrocytes selective MMP-2 and MMP-9 inhibitors were tested to block CD73 being released from interacted astrocytes. As shown in (Figure 3A) and (Figure 3B) MMP-9 inhibitor was capable of completely blocking CD73 being released from interacted astrocytes, while (Figure 3C) demonstrated that an increased amount of the active form of MMP-9 could be detected in the medium of MOG35-55 specifically interacted astrocytes and CD4 effectors.
IV Astrocytes’ CD73 Hindered Pro-Inflammatory Cytokines Production from Interacted CD4 Effectors
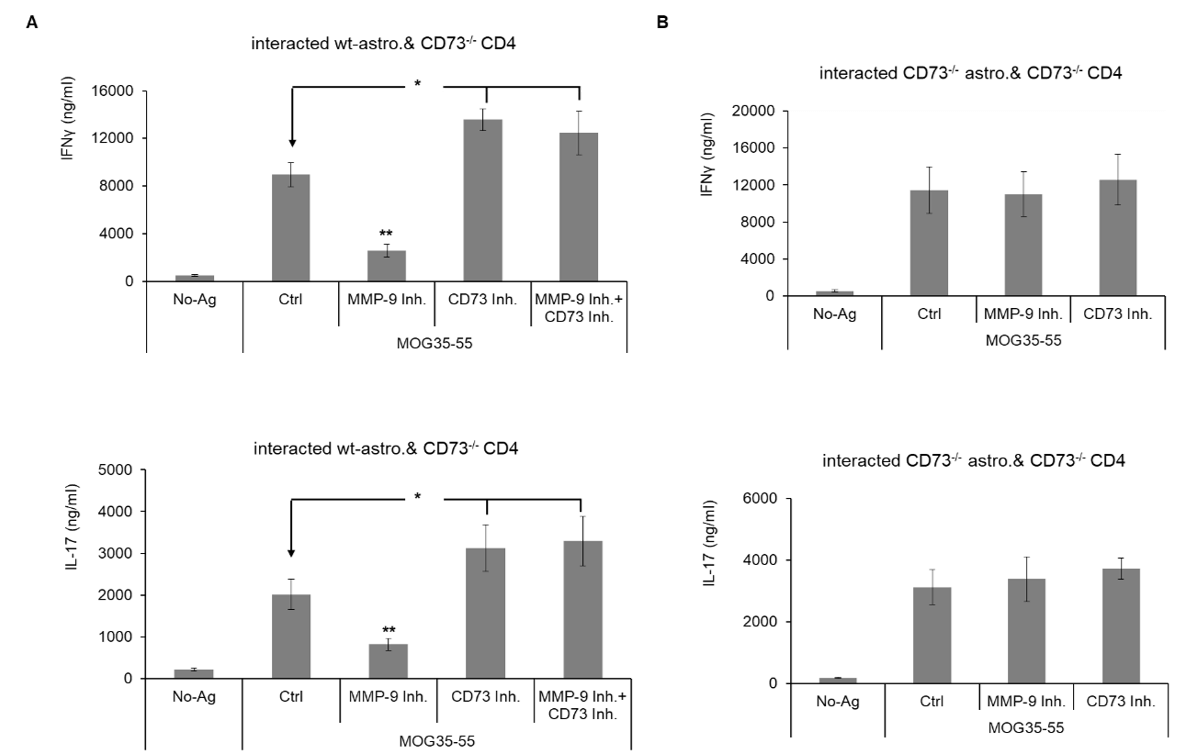
As shown in (Figure 4A), when MMP-9 inhibitor was added to interacted wt-astrocytes and CD73-/- effectors, both IL-17 and IFN-γ productions were significantly decreased, and which could be completely reversed when a CD73 inhibitor was added at the same time. However, for interacted CD73-/-- astrocytes and CD73-/- effectors, the addition of MMP-9 inhibitor did not show any significant effect, shown in (Figure 4B).
Discussion
ATP and its metabolites have strong immune and inflammation regulatory effects. ATP is generally regarded as a pro-inflammatory factor, while adenosine is verified of having a strong anti-inflammatory function. In CNS inflammation, ATP was reported to promote immune response, while adenosine showed significant inhibitory effect [19-24].
By controlling the conversion of AMP to adenosine, CD73 intensively participate in the regulation of immune response, including autoimmune diseases and tumor genesis [25-28]. We had previously reported that under physiological condition, astrocytes are the main cell type in CNS expressing large amounts of CD73 on their surface. When inflammation, such as EAE happened, astrocytes lost most of their membrane-localized CD73. This inhibited the conversion of AMP to adenosine, downregulated adenosine’s immune suppressive function, and promoted local inflammation [8]. In this paper, we try to find out the mechanism leading to downregulated surface CD73 in inflammatory astrocytes.
As shown in (Figure 1), we performed the interaction of astrocytes with CD73-/- CD4 effectors to mimic the condition in CNS when invaded CD4 cells encountered local astrocytes. Firstly astrocytes were primed by the treatment of LPS and IFN-γ to equip them with phagocytosis and antigen presentation function [29]. To avoid the influence of CD4 cells carried CD73, CD73-/- CD4 effectors purified from EAE induced CD73-/- mice were used to interact with primed astrocytes with or without the presence of the specific antigen MOG35-55. As expected, significantly decreased surface CD73 was specifically detected in astrocytes with MOG35-55 was added to the interaction. Only under this condition, the intensive interaction of astrocytes with antigen-specific CD4 effectors is supposed to happen. However, as far as Cd73 mRNA expression was concerned, no significant difference could be detected between different groups of astrocytes. Thus decreased membrane CD73 must be controlled by some mechanisms other than expression regulation. Our first consideration is enzyme controlled CD73 shedding accounting for astrocytes’ CD73 loss. Since CD73 is a GPI anchored membrane protein and PI-PLC may catalyze GPI to release its anchored molecules [30, 31].
To support the idea of CD73 shedding from interacted astrocytes, soluble CD73 molecules is expected to be detected in the medium. The results in (Figure 2) demonstrated the existence of soluble CD73 in the medium, which could be immunoprecipitated and detected by western blot. Interestingly, when compared with membrane extracted CD73, medium immunoprecipitated CD73 (MI-CD73) was revealed to have smaller molecular size, shown in (Figure 2B). C-terminal amino acid sequencing was performed, and MI-CD73 was demonstrated as being C-terminal truncated. The remained first C-terminal amino acid residue is Lys (K), and ‘K547/F548’ is a potential matrix metalloproteinase (MMP) catalyzing site within CD73 molecule.
By means of the addition of selective MMP-2 or MMP-9 inhibitors to MOG35-55 specifically interacted astrocytes and CD4 effectors, it was verified that MMP-9, not MMP-2, is the enzyme accounting for catalyzing CD73 and releasing it from astrocytes’ surface. As shown in (Figures 3A & 3B), when MMP-9 inhibitor was added, the shedding of CD73 from astrocytes’ membrane was almost completely blocked. Meanwhile, significantly elevated amount of active form of MMP-9 was detected in the medium of co-cultured astrocytes and CD4 effectors where downregulated astrocytes’ membrane CD73 happened.
Figure 3: MMP-9 catalyzed CD73 from astrocytes. Selective MMP-2 inhibitor (MMP-2 Inhibitor III, 50 nM) or MMP-9 inhibitor (MMP-9 Inhibitor II, 50 μM) was added to MOG35-55 specific interaction of wt-astrocytes and CD73-/- effectors individually. The surface located CD73 was evaluated by flow cytometry. The active form of MMP-9 in the medium was detected by an MMP-9 Biotrak Activity Assay kit. A) & B) Representative result (A) and statistic result (B, n = 6) of flow cytometry detected CD73 on the surface of interacted astrocytes. C) Amount of active MMP-9 in the media.
Finally, we tested whether the addition of MMP-9 inhibitor to block astrocytes’ CD73 shedding may restore some inhibitory effect of astrocytes to their interacted effectors. Results in (Figure 4A) suggested that when MMP-9 inhibitor was added to MOG35-55 specifically interacted astrocytes and CD4 effectors, the production of IL-17 and IFN-γ, hall markers of Th1 and Th17 cells, were all significantly decreased. When a CD73 inhibitor was added together with the MMP-9 inhibitor, the inhibitory effect from the MMP-9 inhibitor was completely reversed. This strongly indicated that MMP-9 inhibitor exerted its inhibitory effect by maintaining CD73’s activity in astrocytes. To further support this idea, same experiments were performed in interacted CD73-/--astrocytes and CD73-/- effectors. As expected, the addition of MMP-9 inhibitor did not show any effect on inhibiting IL-17 and IFN-γ production. We can confidently say that MMP-9 inhibitor is capable of exerting an anti-inflammatory effect in neuro inflammation by stabilizing CD73 in astrocytes.
Figure 4: Astrocytes’ CD73 hindered pro-inflammatory cytokines production from interacted CD4 effectors. Astrocytes were purified from naïve wt- and CD73-/- B6 mice and subjected to the interaction with CD73-/- EAE-effectors after being primed by LPS (50 ng/ml) and IFN-γ (10 ng/ml), with MOG35-55 present. Interacted cells were treated with MMP-9 inhibitor along with APCP (3 μM), a CD73 inhibitor, or not. IL-17 and IFN-γ in the medium were detected by ELISA. A) IFN-γ (upper column) and IL-17 (lower column) concentrations in the medium of interacted wt-astrocytes and CD73-/- effectors, (n= 6). B) IFN-γ (upper column) and IL-17 (lower column) concentrations in the medium of interacted CD73-/--astrocytes and CD73-/- effectors, (n= 6).
Taken together, it was demonstrated in this paper that it was MMP-9 catalyzing CD73 at its K547/F548 site accounting for CD73 shedding from inflammatory astrocytes. MMP -9 inhibitor could stabilize astrocytes’ membrane CD73 under inflammatory condition and hence exerted suppressive effect to neural inflammation.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81570833).
Article Info
Article Type
Research ArticlePublication history
Received: Sat 06, Jun 2020Accepted: Tue 30, Jun 2020
Published: Wed 22, Jul 2020
Copyright
© 2023 Guoping Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.03.02
Author Info
Yuanyuan Bai Shumin Zhou Jie Guo Fanqiang Kong Song Chen Zhiyun Wang Guoping Liu
Corresponding Author
Guoping LiuDepartment of Neurology, Tianjin first central hospital, Tianjin, China
Figures & Tables

References
- Gennady G Yegutkin (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673-694. [Crossref]
- Marco Idzko, Davide Ferrari, Holger K Eltzschig (2014) Nucleotide signalling during inflammation. Nature 509: 310-317. [Crossref]
- Paul E Zarek, Ching-Tai Huang, Eric R Lutz, Jeanne Kowalski, Maureen R Horton et al. (2008) A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111: 251-259. [Crossref]
- Wolfgang G Junger (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11: 201-212. [Crossref]
- Lesley Ann Smyth, Kulachelvy Ratnasothy, Julia Y S Tsang, Dominic Boardman, Alice Warley et al. (2013) CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol 43: 2430-2440. [Crossref]
- Masahide Takedachi, Dongfeng Qu, Yukihiko Ebisuno, Hiroyuki Oohara, Michelle L Joachims et al. (2008) CD73-generated adenosine restricts lymphocyte migration into draining lymph nodes. J Immunol 180: 6288-6296. [Crossref]
- Francis Coffey, Sang-Yun Lee, Terkild B Buus, Jens-Peter Holst Lauritsen, Gladys W Wong et al. (2014) The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J Exp Med 211: 329-343. [Crossref]
- Cris S Constantinescu, Nasr Farooqi, Kate O'Brien, Bruno Gran (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) . Br J Pharmacol 164: 1079-1106. [Crossref]
- Shumin Zhou, Guoping Liu, Jie Guo, Fanqiang Kong, Song Chen et al. (2019) Pro-inflammatory Effect of Downregulated CD73 Expression in EAE Astrocytes. Front Cell Neurosci 13: 233. [Crossref]
- Ke Dong, Zhao-wei Gao, Hui-zhong Zhang (2016) The role of adenosinergic pathway in human autoimmune diseases. Immunol Res 64: 1133-1141. [Crossref]
- F S Regateiro, S P Cobbold, H Waldmann (2013) CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol 171: 1-7. [Crossref]
- Pavel Chrobak, Roxanne Charlebois, Pavel Rejtar, Rana El Bikai, Bertrand Allard et al. (2015) CD73 plays a protective role in collagen-induced arthritis. J Immunol 194: 2487-2492. [Crossref]
- Sophie Botta Gordon-Smith, Simona Ursu, Simon Eaton, Halima Moncrieffe, Lucy R Wedderburn (2015) Correlation of low CD73 expression on synovial lymphocytes with reduced adenosine generation and higher disease severity in juvenile idiopathic arthritis. Arthritis Rheumatol 67: 545-554. [Crossref]
- Jieyao Li, Liping Wang, Xinfeng Chen, Lifeng Li, Yu Li et al. (2017) CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 6: e1320011. [Crossref]
- Theresa L Whiteside (2017) Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther 17: 527-535. [Crossref]
- Bertrand Allard, Maria Serena Longhi, Simon C Robson, John Stagg (2017) The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 276: 121-144. [Crossref]
- Arabella Young, Shin Foong Ngiow, Deborah S Barkauskas, Erin Sult, Carl Hay et al. (2016) Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell 30: 391-403. [Crossref]
- Stephen D Miller, William J Karpus, Todd Scott Davidson (2010) Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol Chapter 15: Unit-15. 1. [Crossref]
- Geoffrey Burnstock, Gillian E Knight (2018) The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 14: 1-18. [Crossref]
- G Burnstock (2015) Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem 22: 819-844. [Crossref]
- Cristina Ulivieri, Domiziana De Tommaso, Francesca Finetti, Barbara Ortensi, Giuliana Pelicci et al. (2019) A T Cell Suppressive Circuitry Mediated by CD39 and Regulated by ShcC/Rai Is Induced in Astrocytes by Encephalitogenic T Cells. Front Immunol 10: 1041. [Crossref]
- Fabrizio Vincenzi, Carmen Corciulo, Martina Targa, Stefania Merighi, Stefania Gessi et al. (2013) Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated. Eur J Immunol 43: 2206-2216. [Crossref]
- Stefania Gessi, Stefania Merighi, Angela Stefanelli, Debora Fazzi, Katia Varani et al. (2013) A(1) and A(3) adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol Res 76: 157-170. [Crossref]
- Miharu Samuraki, Kenji Sakai, Yasuko Odake, Mitsuhiro Yoshita, Kouichi Misaki et al. (2017) Multiple sclerosis showing elevation of adenosine deaminase levels in the cerebrospinal fluid. Mult Scler Relat Disord 13: 44-46. [Crossref]
- Samaneh Arab, Nasim Kheshtchin, Maryam Ajami, Mahbubeh Ashurpoor, Aida Safvati et al. (2017) Increased efficacy of a dendritic cell-based therapeutic cancer vaccine with adenosine receptor antagonist and CD73 inhibitor. Tumour Biol 39: 1010428317695021. [Crossref]
- Bertrand Allard, Martin Turcotte, John Stagg (2014) Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets 18: 863-881. [Crossref]
- Paul A Beavis, John Stagg, Phillip K Darcy, Mark J Smyth (2012) CD73: a potent suppressor of antitumor immune responses. Trends Immunol 33: 231-237. [Crossref]
- John Stagg, Paul A Beavis, Upulie Divisekera, Mira C P Liu, Andreas Möller et al. (2012) CD73-deficient mice are resistant to carcinogenesis. Cancer Res 72: 2190-2196. [Crossref]
- Cris S Constantinescu, Marie Tani, Richard M Ransohoff, Maria Wysocka, Brendan Hilliard et al. (2005) Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem 95: 331-340. [Crossref]
- Qiujing Shen, Gildas Bourdais, Huairong Pan, Silke Robatzek, Dingzhong Tang (2017) Arabidopsis glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc Natl Acad Sci U S A 114: 5749-5754. [Crossref]
- Calvin Tiengwe, Peter J Bush, James D Bangs (2017) Controlling transferrin receptor trafficking with GPI-valence in bloodstream stage African trypanosomes. PLoS Pathog 13: e1006366. [Crossref]
