Metastatic Anal Lesion of Primary Lobular Breast Carcinoma- An Insidious, Rare and Subclinical Progression: Case Report and Review of the Literature
A B S T R A C T
Gastrointestinal metastasis from primary breast carcinoma is very uncommon, with less than 1% of breast carcinomas metastasize to the gastrointestinal tract but anorectal involvement is even rarer. These metastatic lesions can lead to diagnostic challenge for clinicians as they can mimic primary colo-rectal cancer due to the lack of diagnostic signs. The increase of patient survival could lead to an unusual late resumption of metastatic disease, often for a long-time occult. Nowadays, an almost completely absent literature is unable to suggest diagnostic pathways capable of predicting this type of disease evolution. We present the case of a 76-year old woman who underwent treatment for an infiltrative lobular carcinoma of the breast who developed metastasis of the anal canal 11- years later. A literature review was performed.
Keywords
Breast cancer, metastasis, anal canal, case report
Introduction
Breast cancer is the most common malignancy in women and most frequently metastasizes to the bones, lungs, liver, and brain. Gastrointestinal metastasis from primary breast carcinoma is very uncommon, with less than 1% of breast carcinomas metastasize to the gastrointestinal tract, anorectal involvement is even rarer [1]. Even though Invasive Lobular Carcinoma (ILC) constitutes only 10% of breast cancers, it is the most common breast cancer metastasizing to the colon and rectum [2]. These metastatic lesions can lead to diagnostic challenge for clinicians as they can mimic primary colo-rectal cancer due to the lack of diagnostic signs. Although pathologically distinct, ILC appears to have similar clinical outcomes and prognosis as infiltrating ductal carcinoma (IDC) of the breast. Studies of limited scope suggest that treatment for ILC should follow the guidelines for IDC. However, others suggest that ILC is a different disease that should be treated and followed differently than IDC [3].
A wide literature search on Pubmed was performed applying the keywords: “breast cancer”, “gastrointestinal metastases”, “anorectal metastases and palliative surgery”. Articles and abstracts were also identified by back referencing from original and relevant papers. The thorough literature review revealed only seven individual cases of anal involvement, up to date (Table 1) [4-10].
We present the case of a 76-year old woman who underwent treatment for an infiltrative lobular carcinoma of the left breast with bone metastases and who developed metastasis of the rectum and anal canal 11- years later. We present the following case in accordance with the CARE Guideline.
Case Presentation
In February 2019, a 76-year old woman followed by the Oncological Day Hospital for routine post-treatment follow-ups, was admitted to the Oncology Department with the diagnosis of obstructive acute renal failure. In 2008 she had been diagnosed with a right-sided invasive lobular breast carcinoma pT2N1aM0. Immunohistochemical staining was positive for estrogen receptor (ER 99,6%), progesterone receptor (PR 6,5%) and low for human epidermal growth factor receptor 2 (HER2-neu 1+). The tumor cells showed a low proliferative activity (MIB-1 16,2 %).
Table 1: Reported cases of anal metastasis from breast carcinoma [10].
|
Case |
Age (years) |
Histology |
Interval |
Clinical presentation |
Therapy |
Survival |
|
Dawson et al. [4] |
70 |
ILC |
34 months |
Altering bowel habit, constipation, anal discharge |
Laparotomy and RT |
? |
|
Haberstich et al. [5] |
78 |
IDC |
At diagnosis |
Painful anal tumefaction and blood loss with stools |
Abdominoperineal resection and RT |
Disease-free at 22 months follow-up |
|
Nair et al. [6] |
57 |
IDC |
7 years |
Alternating bowel habit, crampy lower abdominal pain, increased frequency of bowel movements |
Colostomy and RT |
? |
|
Puglisi et al. [7] |
92 |
ILC |
4 years |
Tenesmus and painful anal polypoid lesion |
RT and hormonal therapy |
3 years |
|
Bochicchio et al. [8] |
72 |
ILC |
4 years |
Constipation, tenesmus, fecal incontinence |
Hartmann rectal amputation and RT |
Few months after RT |
|
Rengifo et al. [9] |
78 |
IDC |
27 months before diagnosis of BC |
Rectal bleeding, weight loss, constipation |
RT and hormonal therapy |
? |
|
Ruymbeke et al. [10] |
65 |
ILC |
4 years |
Altering pattern of stool, intermittent fecal incontinence and tenesmus |
RT and hormonal therapy and chemotherapy |
Alive 15 months after diagnosis of anorectal metastasis |
ILC: Invasive Lobular Carcinoma; IDC: Invasive Ductal Carcinoma; HT: Hormonal Therapy; RT: Radiotherapy.
In June 2008 she underwent right-sided upper outer quadrant quadrantectomy with intraoperative assessment of sentinel nodes followed by radical axillary linfoadenectomy. The histological examination showed an infiltrating lobular carcinoma (4x2 mm), grade 2, with metastases in 2 out of 16 nodes removed (pT2N1aM0). She underwent adjuvant chemotherapy according to the FEC regimen (5-fluorouracil, epidoxorubicin and cyclophosphamide), locoregional radiotherapy and hormonal therapy: first with Tamoxifen for 2 years which was replaced by Aromasin for 3 years. In August 2014 she had isolated skin recurrence of the cancer on the right axillary margin of the surgical scar which was surgically removed followed by hormonal therapy with Femara.
She presented 2 years later (2016) with localised low back pain and bone scan revealed two bone metastasis in the Sacrum. This was managed using palliative radiotherapy (single dose 800 cGY) to control the symptoms and her adjuvant hormonal therapy was switched to Fulvestrant and Denosumab. In the later months she had a progression of her metastatic disease. She underwent another cycle of single dose 800 cGY radiotherapy and she started chemotherapy with Exemestane and Everolimus, later switched to Eribulina due to unpleasant physical reaction to the other drug. During this period, she underwent clinical follow-up.
In early 2019, she presented with stranguria and hematuria from several days but no other abdominal symptoms. She also has an ongoing history of chronic stipsis and fourth degree hemorrhoids. Her examination failed to demonstrate any anaemia, jaundice or generalised lymphadenopathy or hematochezia. Examination of her right breast and axillae scars were unremarkable. Blood test results showed an increased Creatinine 4.59 mg/dl. She performed a complete abdomen US that showed bilateral hydroureteronephrosis. She was admitted to the hospital and the Urology team performed a bilateral nephrostomy.
In the following days a staging CT of the head, neck thorax, abdomen and pelvis was performed which revealed a diffuse circumferential wall thickening of the junction rectum-sigma along the anal canal to the perianal region with resultant of an important luminal narrowing and hypervascularization. Marked perirectal fat stranding was observed but except for bone metastases, no other metastatic lesions were identified on the CT scan. Anal and rectal examination was performed by the surgeon and revealed a circumferential induration from h5 to h3 and a complete stenosis of the anal canal. Biopsies were taken and the anatomopathology revealed that they were consistent with metastatic rectal lesions of a primary lobular breast carcinoma. The immunohistochemical staining was identical to the primary breast tumour.
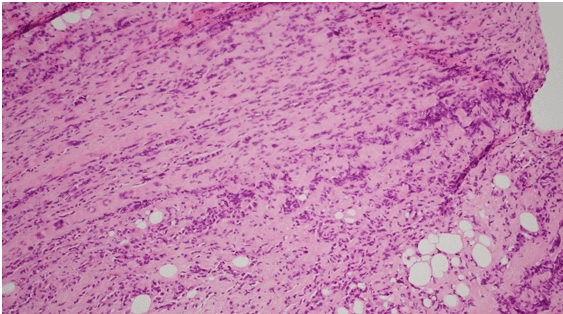
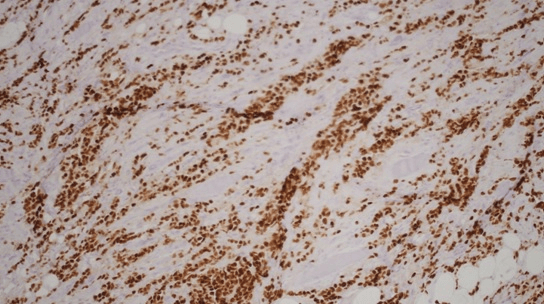
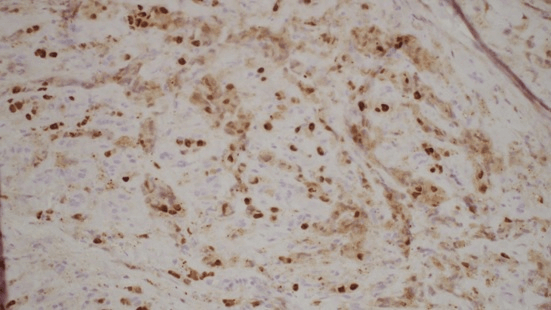
Histology showed fibrotic tissue with invasive carcinoma with cord-like infiltrative growth pattern, typical of breast lobular carcinoma (Figure 1). The tumor cells were positive for estrogen receptor (ER) (Figure 2), progesterone receptor (PR) (Figure 3), and GATA 3 (primarily used as a marker of breast, particularly in metastatic settings). Tumor cells were negative for ECAD. E-cadherin is a calcium-dependent transmembrane protein that mediates cell-cell adhesion and cellular polarity by binding to itself on neighbouring cells in a homophilic manner. In normal breast epithelial cells and in most invasive carcinoma of no special type (IC-NST), E-cadherin is located at the cell membrane, maintaining cellular cohesion. In contrast, approximately 90% of ILCs, including variants, completely lack E-cadherin protein expression. The confirmation of metastatic lobular carcinoma was made on the morphology on the Hematoxylin and eosin section and the ER+/PR+/GATA3+/ECAD - status which was identical to the primary.
Considering the age, the histological diagnosis and the performance status of the patient it was decided to treat the tumor with a systemic chemotherapy with Capecitabina at reduced dose (70%). The patient was later discharged home in discrete clinical conditions followed by the oncologic home care service.
Figure 1: Fibrotic tissue with invasive carcinoma with cord-like infiltrative growth pattern, typical of breast lobular carcinoma.
Figure 2: Tumor cells positive for estrogen receptor (ER).
Figure 3: Progesterone receptor (PR).
Discussion
Gastrointestinal metastases of breast cancer are rare (< 1% in clinical practice) and usually associated with disseminated disease although it is the second most common cancer to metastasize to the GI tract, following malignant melanoma. Metastatic disease to the extrahepatic GIT from breast carcinoma usually originates from the lobular carcinoma subtype which accounts for only 10% of all breast adenocarcinomas, rather than the more common invasive ductal carcinoma. [1] This could be related to a particular tropism of lobular cells to the GIT. It has been well documented that recurrence in lobular breast cancer can occur many years after the initial diagnosis of breast cancer, even in early stage tumors.
Excluding our, only seven individual cases of anal involvement are present in literature so far, four with a history of invasive lobular carcinoma [4, 7, 8, 10] and three with a history of invasive ductal carcinoma [5, 6, 9]. In one case metastasis were discovered during the diagnosis of the primary breast tumor and in another case even 27 months before the diagnosis of the primary breast tumor. The mean time from initial diagnosis to anorectal metastasis is 36 months and the mean age of patients is 73 years. In our case, GIT recurrence occurred 11 years after treatment of pT2N1aM0 breast cancer. Recurrences of lobular breast cancer have been reported up to 30 years after the initial diagnosis [10]. The combination of its rarity, the often unusually long interval and the non-specific clinical presentation makes the diagnosis of GI metastasis from primary breast carcinoma a challenge. However, due to the unique characteristics of ILC, some authors propose a closer follow up incorporating a strategy for GI tract evaluation to be implemented for patients with ILC [3].
Symptoms depend on the localization of metastatic disease, such as diarrhea, constipation, obstruction, incontinence, weight loss, rectal bleeding and tenesmus. Most symptoms are non-specific and may be considered treatment-related or as inflammatory GI disease. In our case the discovery of the metastatic tumor was accidental, considering the fact that our patient did not have any of the symptoms that we would normally encounter, she suffered of chronic stipsis and forth degree hemorrhoids which could have delayed the diagnosis that was firstly seen at the CT scan. Histopathological diagnosis of metastatic lesions can be challenging as well. The lack of dysplasia of the rectal mucosa is often helpful in distinguishing between a primary and metastatic lesion. Immunohistochemical techniques will allow for the most accurate diagnosis. ER, PR and HER2-neu status should be compared with the features of the primary breast tumor [12].
Systemic treatment with chemotherapy and/or hormonal therapy is usually employed in patients with confirmed diagnosis of gastrointestinal metastasis [4]. The role of surgery is limited to palliation or for patients presenting acutely with obstruction or perforation of the hollow viscus. Among the performed palliative procedures there is diverting colostomy and in one case, a year after starting treatment with chemotherapy for metastasis, the patient had closure of colostomy [13]. The prognosis of GI metastasis from primary breast cancer is poor with few patients surviving beyond two years, although survival up to nine years has been reported. Given the increased survival of breast cancer patients due to the early diagnosis through breast screening and better management with current therapeutic regimens, more unusual presentations of metastatic disease, including involvement of the gastrointestinal tract should be anticipated. Probably new diagnostic pathways will be needed in the future, by customizing the follow-up.
Article Info
Article Type
Case Report & Review of LiteraturePublication history
Received: Mon 09, Mar 2020Accepted: Sat 28, Mar 2020
Published: Mon 30, Mar 2020
Copyright
© 2023 Fabbri Nicolò. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.03.12
Author Info
Aisoni F Bonazza S Carcoforo P D’Urbano F Fabbri Nicolò Pedriali M Soliani G
Corresponding Author
Fabbri NicolòUO di Chirurgia Generale Provinciale, Azienda USL di Ferrara, Via Valle Oppio 2, Lagosanto, Italy
Figures & Tables
Table 1: Reported cases of anal metastasis from breast carcinoma [10].
|
Case |
Age (years) |
Histology |
Interval |
Clinical presentation |
Therapy |
Survival |
|
Dawson et al. [4] |
70 |
ILC |
34 months |
Altering bowel habit, constipation, anal discharge |
Laparotomy and RT |
? |
|
Haberstich et al. [5] |
78 |
IDC |
At diagnosis |
Painful anal tumefaction and blood loss with stools |
Abdominoperineal resection and RT |
Disease-free at 22 months follow-up |
|
Nair et al. [6] |
57 |
IDC |
7 years |
Alternating bowel habit, crampy lower abdominal pain, increased frequency of bowel movements |
Colostomy and RT |
? |
|
Puglisi et al. [7] |
92 |
ILC |
4 years |
Tenesmus and painful anal polypoid lesion |
RT and hormonal therapy |
3 years |
|
Bochicchio et al. [8] |
72 |
ILC |
4 years |
Constipation, tenesmus, fecal incontinence |
Hartmann rectal amputation and RT |
Few months after RT |
|
Rengifo et al. [9] |
78 |
IDC |
27 months before diagnosis of BC |
Rectal bleeding, weight loss, constipation |
RT and hormonal therapy |
? |
|
Ruymbeke et al. [10] |
65 |
ILC |
4 years |
Altering pattern of stool, intermittent fecal incontinence and tenesmus |
RT and hormonal therapy and chemotherapy |
Alive 15 months after diagnosis of anorectal metastasis |
ILC: Invasive Lobular Carcinoma; IDC: Invasive Ductal Carcinoma; HT: Hormonal Therapy; RT: Radiotherapy.
References
- Borst MJ, Ingold JA (1993) Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 114: 637- 641. [Crossref]
- Schwarz RE, Klimstra DS, Turnbull AD (1998) Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol 93: 111-114. [Crossref]
- Carcoforo P, Raiji MT, Langan RC, Lanzara S, Portinari M et al. (2012) Infiltrating lobular carcinoma of the breast presenting as gastrointestinal obstruction: a mini review. J Cancer 3: 328-332. [Crossref]
- Dawson PM, Hershman MJ, Wood CB (1985) Metastatic carcinoma of the breast in the anal canal. Postgrad Med J 61: 1081. [Crossref]
- Haberstich R, Tuech JJ, Wilt M, Rodier JF (2005) Anal localization as first manifestation of metastatic ductal breast carcinoma. Tech Coloproctol 9: 237- 238. [Crossref]
- Nair M, Fafemi O (2007) Anorectal metastasis from breast carcinoma: a case report with literature review. Internet J Oncol 5.
- Puglisi M, Varaldo E, Assalino M, Ansaldo G, Torre G et al. (2009) Anal metastasis from recurrent breast lobular carcinoma: a case report. World J Gastroenterol 15: 1388-1390. [Crossref]
- Bochicchio A, Tartarone A, Ignomirelli O, Latorre G, Cangiano R et al. (2012) Anal metastasis from breast cancer: a case report and review of the literature. Future Oncol 8: 333- 336. [Crossref]
- Rengifo C, Titi S, Walls J (2016) Anal metastasis as the sentinel and isolated presentation of invasive ductal breast carcinoma. Ann R Coll Surg Engl 98: e68-e70. [Crossref]
- Ruymbeke H, Harlet L, Stragier B, Steenkiste E, Ryckx M et al. (2018) Anorectal metastasis from breast carcinoma: a case report and review of the literature. BMC Res Notes 11: 268. [Crossref]
- Benfiguig A, Anciaux ML, Eugène CI Benkémoun G, Etienne JC (1992) [Gastric metastasis of breast cancer occurring after a cancer-free interval of 30 years] (article in French). Ann Gastroenterol Hepatol (Paris) 28: 175-177. [Crossref]
- Arrangoiz R, Papavasiliou P, Dushkin H, Farma JM (2011) Case report and literature review: metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep 2: 301-305. [Crossref]
- Lau LC, Wee B, Wang S, Thian YL (2017) Metastatic breast cancer to the rectum: A case report with emphasis on MRI features. Medicine (Baltimore) 96: e6739. [Crossref]
