MDCT Evaluation of Hepatic Manifestations in Malignant Hematological Disorders
MDCT Evaluation of Hepatic Manifestations in Malignant Hematological Disorders
A B S T R A C T
Objective: The aim of this work was to evaluate Multidetector Computed Tomography (MDCT) findings of hepatic manifestation in common malignant hematological disorders.
Materials and Methods: This retrospective study included 300 patients with different types of malignant hematological disorders. They were 119 female and 181 male (mean age, 45.4 year; range, 5-70 years). The most common hematological disorder was Non-Hodgkin lymphoma (NHL) detected in 192/300 (64%) patients and then Hodgkin disease (HD) detected in 40/300 (13.3%) patients. All 300 patients had ultrasonography. Clinical evaluation and laboratory assessment were done for all patients. Whole body and triphasic abdominal CT scanning was performed on 64 MDCT systems.
Results: MDCT scanning revealed hepatic affection in 82/192 of NHL. All these 82 cases revealed hepatomegaly and focal lesion detected in 36 cases. As regard Hodgkin disease (HD), hepatomegaly detected in 22/40 cases and focal lesion detected in 8/40 cases. Hepatomegaly detected in 8/14 cases of acute myeloid leukemia (AML) and focal lesion detected in 4/14 cases. In cases of chronic lymphocytic leukemia (CLL), hepatomegaly detected in 6/22 cases with no detection of focal lesion.
Conclusion: Hepatic involvement is often observed in several malignant hematological disorders, resulting in abnormalities in liver imaging studies. Malignant hematological disorders must be considered in hepatomegaly and hepatic focal lesions either single or multiple. MDCT is the diagnostic modality of choice for diagnosis and follow up of liver affection in different malignant hematological disorders.
Keywords
Liver, hematological malignancies, computed tomography
Introduction
Liver involvement is often observed in several malignant hematological disorders, resulting in abnormal liver function tests, abnormalities in liver imaging studies, or clinical symptoms presenting with hepatic manifestations. In hemolytic anemia, jaundice and hepatosplenomegaly are often seen mimicking liver diseases. In hematologic malignancies, malignant cells often infiltrate the liver and may demonstrate abnormal liver function test results accompanied by hepatosplenomegaly or formation of multiple nodules in the liver and/or spleen. These cases may further evolve into fulminant hepatic failure [1]. Hepatologists or general physicians sometimes encounter hepatic manifestations of various hematologic disorders in daily practice, including various abnormalities in liver function tests or imaging studies of the liver. Some hematologic disorders also mimic liver diseases [2].
The prominent role of computed tomography multidetector is primarily defined by its excellent ability to depict morphologic characteristics, in particular in clinical scenarios of diffuse or focal intrahepatic lesions as well as anatomic relationships between the liver and adjacent organs [3, 4]. In the present study, we aimed to evaluate multidetector Computed Tomography findings of hepatic manifestation in common malignant hematological disorders.
Materials and Methods
I Patients
The study was approved by the institutional research ethics review committee. This retrospective study included 300 patients with different types of hematological disorders between January 2016 and October 2018. They were 119 female and 181 male (mean age, 45.4 year; range, 5-70 years). The most common hematological disorder was Non-Hodgkin lymphoma (NHL) detected in 192/300 (64%) patients and then Hodgkin disease (HD) detected in 40/300 (13.3%) patients. All 300 patients had ultrasonography. Exclusion criteria were patients with cirrhotic liver, Child-Pugh B and C classification, patients undergo surgical intervention in liver or patients who undergo hepatic resection. All patients were subjected to history taking, complete general and local examination as well as clinical grading of malignant lesion. The most common clinical presentation was abdominal pain detected 120/300 (40%) and second common presentation was neck swelling detected in 48/300 (16%). Progressive loss of weight detected in 30/300 (10%) patient. Palpable abdominal masses detected in 26/300 (8.7%). Laboratory abnormalities were detected in all cases. These abnormalities on admission showed the following results: mild elevated total bilirubin 0.8-5 mg/dL (normal 0.1-1.1), aspartate aminotransferase (AST) 40- 170 IU/mL (up to 40) and alanine aminotransferase (ALT) 35- 150 IU/L (up to 40). Fine needle aspiration was done from hepatic focal lesion for pathological evaluation.
II Methods
i CT Technique
Whole body and triphasic abdominal CT scanning was performed on 64 MDCT system (Brilliance 64; Philips Healthcare, Best, The Netherlands). The precontrast and post-contrast series were taken by using a 5 mm slice thickness. Arterial and delayed phases were done for abdominal examination. Portal phase was done for whole body. The post-contrast study was performed using low osmolar non-ionic contrast medium (Ioversol, Optiray 350). The dose was adjusted according to body weight. Patients were requested to hold their breath during the precontrast phase and the three phases of acquisition. Automated bolus tracking with bolus detection at the level of the descending aorta above the diaphragm ensured accurate timing of the data acquisition in an arterial phase. Portal venous phase was performed with an effective delay of 55-60 seconds after initiation of the contrast material injection. The delayed phase was performed with effective delay of 3-6 minutes. All images were transferred to the workstation [Extended Brilliance Workspace V3.5.0.2254] (EBW) for post processing. The images were viewed on lung, soft tissue and bone setting.
ii Image Interpretation
Data interpretation and image analysis focused on the following aspects on the initial CT scan: hepatic size, pre-contrast hepatic attenuation, lesion attenuation, density in all phases (arterial, portal and delayed phases), number of lesions, vascular invasion, lymph node involvement and other abdominal organs as well as metastatic spread. Whole body CT scanning was evaluated for all groups of lymph nodes and other organs of the body as well as bony or pulmonary metastases. Tumor size, internal architecture, organ of origin, tissue invasion, vascular encasement, calcifications and metastases were evaluated. Tumor staging of all malignancies was evaluated.
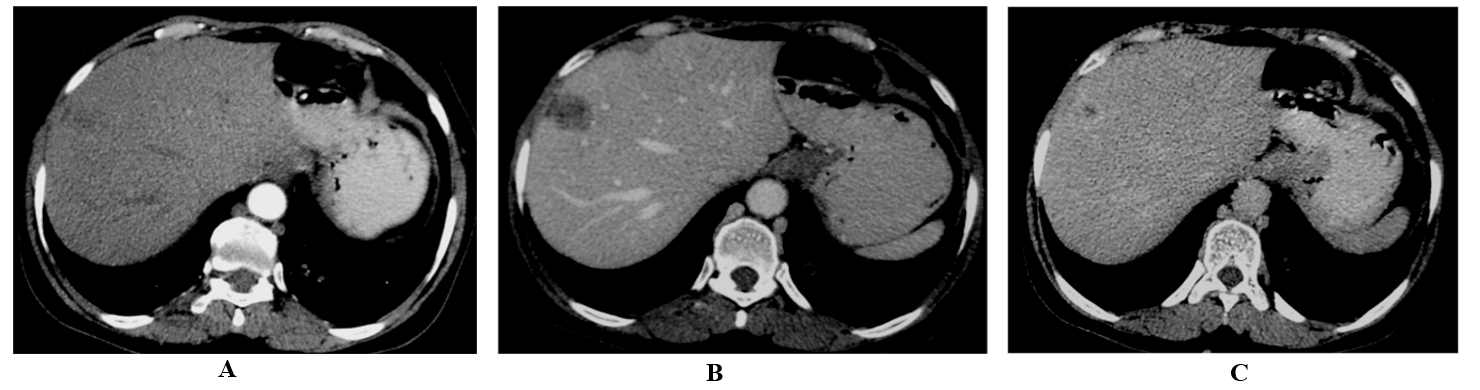
Figure 1: 46-year-old man with abdominal pain and elevated lactate dehydrogenase (LDH). Pathologically proved Non-Hodgkin lymphoma. Multi-detector triphasic CT scan of the abdomen during the A) arterial phase, B) portal phase and C) delayed phase shows a focal hepatic lesion in segment VIII with delayed enhancement.
Results
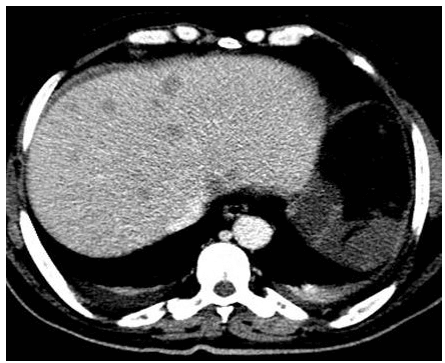
MDCT scanning revealed hepatic affection in 82/192 (42.7%) cases of Non-Hodgkin lymphoma (NHL). All these 82 revealed hepatomegaly and focal lesion detected in 36 (18.7%) cases (Figure 1). All focal lesions were confirmed pathologically, and they were of the same hematological malignancy. As regard Hodgkin disease (HD), hepatomegaly detected in 22/40 (55%) cases and focal lesion detected in 8/40 (20%) cases (Figure 2).
Figure 2: 6-year-old female presented with fever and weight loss. Post-contrast CT scan of abdomen revealed multiple hypodense non-enhancing hepatic focal lesions and multiple enlarged portahepatis, para-aortic, retro-caval and splenic hilar lymphadenopathy. Pathologically proved to be Hodgkin disease.
No cases with primary hepatic lymphoma detected. Hepatomegaly detected in 8/14 (57.1%) cases of acute myeloid leukemia (AML) and focal lesion detected in 4/14 (28.6%) cases. No detected calcifications of hepatic focal lesions. Hepatomegaly detected in 6/14 (42.85%) cases of acute lymphoblastic leukemia (ALL) and focal lesion detected in 2/14 (14.29%) cases (Figure 3). In cases of chronic lymphocytic leukemia (CLL), hepatomegaly detected in 6/22 (27.3%) cases with no detection of focal lesion. Hepatic affection of 300 cases of malignant hematological disorders was showed in (Tables 1 & 2). The associated extra-hepatic pathological findings were showed in (Table 3).
Figure 3: 50-year-old female patient presented with fever and old history of splenectomy. Laboratory investigation demonstrates pancytopenia. Pathology proved to be acute lymphoblastic leukemia. Post-contrast CT scan of abdomen revealed multiple well defined hypodense non-enhancing focal lesions on both lobes mild ascites.
Table 1: Hepatic affection in 300 cases of different hematological disorders.
|
Hematological malignancies |
No. of cases |
No detected Hepatic findings |
Hepatic findings |
|
|
Enlarged |
Focal lesion |
|||
|
NHL |
192 |
110 (57.3%) |
82 (42.7%) |
36 (18.75%) |
|
HD |
40 |
18 (45%) |
22 (55%) |
8 (20%) |
|
Follicular lymphoma |
6 |
2 (33.3%) |
4 (66.7%) |
2 (33.3%) |
|
Anaplastic large cell lymphoma |
2 |
- |
2 (100%) |
- |
|
Lymphoblastic Lymphoma. |
4 |
- |
4 (100%) |
2 (50%) |
|
Hairy cell Leukaemia |
6 |
4 (66.7%) |
2 (33.3%) |
- |
|
AML |
14 |
6 (42.9%) |
8 (57.1%) |
4 (28.6%) |
|
CLL |
22 |
14 (63.63%) |
8 (36.36%) |
- |
|
ALL |
14 |
8 (57.1) |
6 (42.85%) |
2 (14.29%) |
|
Total |
300 (100%) |
162 (54%) |
138 (46%) |
54 (18%) |
ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.
Table 2: Characterizations of focal lesions in 54 cases of different hematological disorders.
|
Hematological malignancies |
No. of cases |
Multiplicity |
Pattern of enhancement in Post-contrast CT |
||
|
Single |
Multiple |
Non-enhanced |
Enhanced |
||
|
NHL |
36 |
14 |
22 |
33 |
3 |
|
HD |
8 |
6 |
2 |
7 |
1 |
|
Follicular lymphoma |
2 |
2 |
- |
2 |
- |
|
Anaplastic large cell lymphoma |
- |
- |
- |
- |
- |
|
Lymphoblastic Lymphoma. |
2 |
2 |
- |
2 |
- |
|
Hairy cell Leukaemia |
- |
- |
- |
- |
- |
|
AML |
4 |
4 |
- |
4 |
- |
|
CLL |
- |
- |
- |
- |
- |
|
ALL |
2 |
- |
2 |
2 |
- |
|
Total |
54 |
28 |
26 |
50 |
4 |
ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.
Table 3: Most common associated pathological findings in those patients in post contrast abdominal CT.
|
|
Total No. |
Abd. LN |
Pelvic LN |
Splen.+ |
Splenic Fl. |
Paraspinal mass |
|
NHL |
192 |
152 |
90 |
130 |
80 |
24 |
|
HD |
40 |
24 |
30 |
28 |
14 |
- |
|
Follicular lymphoma |
6 |
6 |
4 |
4 |
2 |
2 |
|
Anaplastic large cell lymphoma |
2 |
2 |
2 |
2 |
2 |
- |
|
Lymphoblastic Lymphoma. |
4 |
4 |
4 |
2 |
2 |
- |
|
Hairy cell Leukaemia |
6 |
1 |
1 |
5 |
- |
- |
|
AML |
14 |
8 |
8 |
6 |
- |
- |
|
CLL |
22 |
18 |
13 |
12 |
- |
4 |
|
ALL |
14 |
10 |
8 |
8 |
- |
- |
|
Total |
300 (100%) |
225 (75%) |
160 (53.3%) |
197 (65.7%) |
100 (33.3%) |
30 (10%) |
Abd. LN: Abdominal Lymphadenopathy; Pelvic LN: Pelvic Lymphadenopathy; Splen.+: Splenic Enlargement; Splenic FL: Splenic Focal Lesion; ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.
Discussion
Extranodal lymphoproliferative diseases are common, and their prevalence is increasing. Familiarity with imaging findings that are diagnostically specific for extra-nodal lymphoproliferative disorders is important because imaging plays an important role in the noninvasive management of disease. However, a definitive diagnosis requires a biopsy, peripheral blood analysis, and other laboratory tests [5].
Hepatic hematologic malignancies include a wide spectrum of lymphoproliferative and myeloproliferative disorders. In many cases, knowledge of the clinical manifestations and imaging findings will raise concern for a hematologic disease involving the liver. Criteria that suggest hematologic disease include patient age younger than 40 years, B symptoms of lymphoma, multiple liver lesions with no known history of cancer, hepatosplenomegaly or concurrent splenic lesions, vascular encasement without occlusion or thrombosis, and widespread adenopathy above and below the diaphragm. The possibility of a hematologic disorder may change management and obviate surgery. If biopsy is contemplated, core biopsy may be required instead of fine-needle aspiration to increase the possibility of correct diagnosis [6]. Lymphomatous involvement of liver can manifest on imaging as solitary or multiple nodular lesions, diffuse infiltration, or as a periportal soft tissue mass [6, 7].
The malignant lymphomas, Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL), account for approximately 5-6% of all malignancies [8]. Lymphomas frequently involve nodal and extranodal structures in the abdomen and pelvis [9]. HD is usually almost entirely confined to the lymph nodes [10]. Lee et al., 2008 reported that extranodal lymphoma occurs in about 40% of patients and has been described in virtually every organ and tissue. In decreasing order of frequency, the spleen and liver are most affected [9]. Liver may be an anatomical site for both primary and secondary lymphoma. Similar to the systemic lymphoma, our study demonstrated that Hodgkin disease (HD) consists of a heterogeneous group of lymphoproliferative disorders. The clinical presentation, laboratory and radiology results of Hodgkin disease (HD) are often ambiguous. The awareness of this entity and early application of histopathology evaluation in patients presenting with intrahepatic mass or mass-like lesion will ultimately improve clinical outcomes [11].
Liver involvement has been reported in 14% of patients with Hodgkin disease (HD). Hepatomegaly is found in 9% of patients with disease stages I-II and in 45% of patients with stages III-IV. Mild elevation of aminotransferase and moderate elevation of ALP can occur due to tumor infiltration or extrahepatic bile duct obstruction [12]. Cholestasis can be caused by direct infiltration of lymphoma cells, extrahepatic biliary obstruction, viral hepatitis, drug hepatotoxicity, or vanishing bile duct syndrome. Approximately 3-13% of patients with Hodgkin disease (HD) present with jaundice [13]. Acute liver failure can be caused by ischaemia secondary to compression of the hepatic sinusoids by infiltrating lymphoma cells Hodgkin disease (HD) [14]. NHL has been classified by cell morphology as small to large cell type and according to the natural history of the clinical aggressiveness of the disease as low, intermediate or high grade [1].
Lymphoma cell infiltration of the liver with hepatomegaly is more common in NHL than in HD, with 16-43% of cases showing hepatic involvement [12]. Acute liver failure due to lymphoma can be suspected in cases of acute onset of hepatic enlargement and lactic acidosis different from other causes of liver failure [15, 16]. Our study in agreement with Roos and Friedman 2006 as regard NHL [12]. In our study we find that liver affection occur in 42.7% of cases with NHL and 55% of cases with HD. The difference of HD; may be due small number of our cases. Discrete nodules occur in about 10% of cases of Hodgkin disease and non-Hodgkin lymphomas of the liver and may appear with a miliary pattern [5]. Both primary and secondary hepatic lymphomas are more often non-Hodgkin lymphoma than they are Hodgkin disease. The liver is involved in up to 15% of patients with non-Hodgkin lymphoma and in up to 10% of patients with Hodgkin disease [5]. Our study revealed focal lesion in 36/192 (18.8%) cases in NHL & 8/40 (20%) cases in HD.
In general, lymphomatous involvement of solid organs as liver may occur as a focal, multifocal, or diffuse disease processes. Uniform (solitary) or multifocal (multiple) nodules are round, well defined, and homogeneous. Calcification is rare in the absence of treatment. On CT scans, nodules commonly are hypoattenuating when compared with the surrounding organ parenchyma but have attenuation values higher than that of water [5, 17, 18]. These coincide with our results as 50/54 (92.6%) of cases with focal lesions are non-enhanced hypo attenuated and no detected calcifications in all focal lesions. Involvement of the liver occurs up to 15% (6-20%) of patients with lymphoma and may be focal or diffuse with or without hepatomegaly. It is usually diffuse, with discrete nodular lesions being present in only 10% [9].
Leukemia cells often infiltrate the liver, in both the portal tracts and sinusoids, and liver enlargement has been observed in up to 40% of patients [1]. Our study showed 50 cases of AML, CLL and ALL. Of them 14/50 showed hepatomegaly. The difference between this previous study and our results may be due to small number of our cases.
There are two main types of leukemia, acute leukemia (AL) and chronic leukemia (CL). AL includes acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML) [19]. Acute lymphoblastic leukemia (ALL) is associated with decrease in normal blood cells caused by replacement of the bone marrow with tumor cells. The clinical presentations of patients include anemia, bleeding tendency, or susceptibility to infections. B-symptoms such as fever, night sweats, and weight loss are often present but may be mild. Hepatomegaly, splenomegaly or lymphadenopathy can be seen in up to half of the adult patients upon presentation [1]. Palpable organomegaly as a presentation of AML is uncommon, and significant lymph node enlargement is rare in patients with AML. Marked hepatosplenomegaly is also uncommon; however, if present, the patient is likely to have ALL or evolution of AML from a prior myeloproliferative disorder (blast crisis of CML) [1].
Extramedullary involvement is considered to be an uncommon presentation of AL. However, some data suggest it may be present in up to 30% of patients with AML and has been increasingly reported in ALL patients [20-22]. Our study revealed hepatomegaly in 8/14 (57.1%) cases of AML and hepatic focal lesion in 4/14 (28.6%) cases. In cases of ALL, our study revealed hepatomegaly in 4/14 cases and hepatic focal lesion in 2/14 cases.
Clinical presentation of hairy cell leukemia (HCL) includes the following: 1) abdominal fullness due to splenomegaly, which may cause spontaneous splenic rupture, 2) systemic symptoms such as fatigue, weakness, and weight loss without fever or night sweats, 3) bleeding tendency secondary to severe thrombocytopenia or recurrent infections, and 4) asymptomatic splenomegaly or cytopenias which may be incidentally recognized, and the most common physical sign of HCL is palpable splenomegaly (80%-90% of cases) [23]. Massive splenomegaly extending more than 8 cm below the left costal margin is observed in 25% of cases. Hepatomegaly and lymphadenopathy are not common in HCL, presenting about 20% and 10% of patients respectively. Our result revealed two cases (2/6) (33%) with hepatomegaly.
Conclusion
Hepatic involvement is often observed in several malignant hematological disorders, resulting in abnormalities in liver imaging studies. Malignant hematological disorders must be considered in hepatomegaly and hepatic focal lesions either single or multiple. MDCT is the diagnostic modality of choice for diagnosis and follow up of liver affection in different malignant hematological disorders.
Abbreviations
AL: Acute Leukemia
ALL: Acute Lymphoblastic Leukemia
AML: Acute Myeloid Leukemia
CL: Chronic Leukemia
CLL: Chronic Lymphocytic Leukemia
CML: Chronic Myeloid Leukemia
HCL: Hairy Cell Leukemia
HD: Hodgkin Disease
HL: Hodgkin Lymphoma
MDCT: Multi-Detector Computed Tomography
NHL: Non-Hodgkin Lymphoma
Article Info
Article Type
Research ArticlePublication history
Received: Thu 29, Oct 2020Accepted: Mon 09, Nov 2020
Published: Thu 19, Nov 2020
Copyright
© 2023 Adel El-Badrawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CDM.2020.02.01
Author Info
Adel El-Badrawy Ahmed Tawfik Gehad Saleh Mai Abdel-Naby Hayam Ghazy Ziad Emarah Ahmed Ramez Mohamed A. Elfatah Ahmed Eltantawy Mona M. Taalab Noha Eisa Shaimaa El-Ashwah Yasmine Essam Basma Atef Shahira Ali El-Etreby Monir H. Bahgat Ahmed Deiab Hatem Elalfy Tarek Besheer Ahmed El-Mesery Mohamed Mahmoud Sarhan Suzy Abd Elmabood Ahmed Megahed Mohamed Farouk Akl Khaled Abouelkhair Hanan Wahba Amal Halim Nirmeen Megahed Eman Omar Khashaba
Corresponding Author
Adel El-BadrawyRadiology Department, Mansoura Faculty of Medicine, Mansoura University, Egypt
Figures & Tables
Table 1: Hepatic affection in 300 cases of different hematological disorders.
|
Hematological malignancies |
No. of cases |
No detected Hepatic findings |
Hepatic findings |
|
|
Enlarged |
Focal lesion |
|||
|
NHL |
192 |
110 (57.3%) |
82 (42.7%) |
36 (18.75%) |
|
HD |
40 |
18 (45%) |
22 (55%) |
8 (20%) |
|
Follicular lymphoma |
6 |
2 (33.3%) |
4 (66.7%) |
2 (33.3%) |
|
Anaplastic large cell lymphoma |
2 |
- |
2 (100%) |
- |
|
Lymphoblastic Lymphoma. |
4 |
- |
4 (100%) |
2 (50%) |
|
Hairy cell Leukaemia |
6 |
4 (66.7%) |
2 (33.3%) |
- |
|
AML |
14 |
6 (42.9%) |
8 (57.1%) |
4 (28.6%) |
|
CLL |
22 |
14 (63.63%) |
8 (36.36%) |
- |
|
ALL |
14 |
8 (57.1) |
6 (42.85%) |
2 (14.29%) |
|
Total |
300 (100%) |
162 (54%) |
138 (46%) |
54 (18%) |
ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.
Table 2: Characterizations of focal lesions in 54 cases of different hematological disorders.
|
Hematological malignancies |
No. of cases |
Multiplicity |
Pattern of enhancement in Post-contrast CT |
||
|
Single |
Multiple |
Non-enhanced |
Enhanced |
||
|
NHL |
36 |
14 |
22 |
33 |
3 |
|
HD |
8 |
6 |
2 |
7 |
1 |
|
Follicular lymphoma |
2 |
2 |
- |
2 |
- |
|
Anaplastic large cell lymphoma |
- |
- |
- |
- |
- |
|
Lymphoblastic Lymphoma. |
2 |
2 |
- |
2 |
- |
|
Hairy cell Leukaemia |
- |
- |
- |
- |
- |
|
AML |
4 |
4 |
- |
4 |
- |
|
CLL |
- |
- |
- |
- |
- |
|
ALL |
2 |
- |
2 |
2 |
- |
|
Total |
54 |
28 |
26 |
50 |
4 |
ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.
Table 3: Most common associated pathological findings in those patients in post contrast abdominal CT.
|
|
Total No. |
Abd. LN |
Pelvic LN |
Splen.+ |
Splenic Fl. |
Paraspinal mass |
|
NHL |
192 |
152 |
90 |
130 |
80 |
24 |
|
HD |
40 |
24 |
30 |
28 |
14 |
- |
|
Follicular lymphoma |
6 |
6 |
4 |
4 |
2 |
2 |
|
Anaplastic large cell lymphoma |
2 |
2 |
2 |
2 |
2 |
- |
|
Lymphoblastic Lymphoma. |
4 |
4 |
4 |
2 |
2 |
- |
|
Hairy cell Leukaemia |
6 |
1 |
1 |
5 |
- |
- |
|
AML |
14 |
8 |
8 |
6 |
- |
- |
|
CLL |
22 |
18 |
13 |
12 |
- |
4 |
|
ALL |
14 |
10 |
8 |
8 |
- |
- |
|
Total |
300 (100%) |
225 (75%) |
160 (53.3%) |
197 (65.7%) |
100 (33.3%) |
30 (10%) |
Abd. LN: Abdominal Lymphadenopathy; Pelvic LN: Pelvic Lymphadenopathy; Splen.+: Splenic Enlargement; Splenic FL: Splenic Focal Lesion; ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; CLL: Chronic Lymphocytic Leukemia; HD: Hodgkin Disease; NHL: Non-Hodgkin Lymphoma.

References
- Murakami J, Shimizu Y (2013) Hepatic manifestations in hematological disorders. Int J Hepatol 2013: 484903. [Crossref]
- Singh MM, Pockros PJ (2011) Hematologic and oncologic diseases and the liver. Clin Liver Dis 15: 69-87. [Crossref]
- Boll DT, Merkle EM (2009) Diffuse Liver Disease: Strategies for Hepatic CT and MR Imaging. Radiographics 29: 1591-1614. [Crossref]
- Zhang S, Xie SM, Chen YH, Liu XB, Mai G et al. (2017) Distinct MDCT imaging features to differential diagnosis of hepatic paragnonimiasis and small hepatocellular carcinoma. Oncotarget 8: 37291-37295. [Crossref]
- Leite NP, Kased N, Hanna RF, Brown MA, Pereira JA et al. (2007) Cross-sectional imaging of extranodal involvement in abdominopelvic lymphoproliferative malignancies. Radiographics 27: 1613-1634. [Crossref]
- Tomasian A, Sandrasegaran K, Elsayes KM, Shanbhogue A, Shaaban A et al. (2015) Hematologic malignancies of the liver: spectrum of disease. Radiographics 35: 71-86. [Crossref]
- Abe H, Kamimura K, Kawai H, Kamimura H, Domori K et al. (2014) Diagnostic imaging of hepatic lymphoma. Clin Res Hepatol Gastroenterol 39: 435-442 [Crossref]
- Kwee TC, Kwee RM, Nievelsteinv RAG (2008) Imaging in staging of malignant lymphoma: a systematic review. Blood 111: 504-516. [Crossref]
- Lee WK, Lau EWF, Duddalwar VA, et al. (2008) Abdominal manifestations of extranodal lymphoma: spectrum of imaging findings. AJR Am J Roentgenol 191: 198-206. [Crossref]
- Metser U, Goor O, Lerman H, Naparstek E, Even Sapir E (2004) PET-CT of extranodal lymphoma. AJR Am J Roentgenol 182: 1579-1586. [Crossref]
- Bai YF, Liu JM, Zhang XM, Jiang CZ, Xu X et al. (2017) Percutaneous liver biopsy: retrospective study of primary and secondary hepatic lymphoma in twenty-one patients. Hepatobiliary Pancreat Dis Int 16: 58-64. [Crossref]
- Ross A, Friedman LS (2006) The liver in systemic disease, in Comprehensive Clinical Hepatology, 2nd edition. Mosby Elsevier, Philadelphia, Pa, USA 537.
- Guliter S, Erdem O, Isik M, Yamac K, Uluoglu O (2004) Cholestatic liver disease with ductopenia (vanishing bile duct syndrome) in Hodgkin's disease: report of a case. Tumori 90: 517-520. [Crossref]
- Rowbotham D, Wendon J, Williams R (1998) Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut 42: 576-580. [Crossref]
- Shimizu Y (2008) Liver in systemic disease. World J Gastroenterol 14: 4111-4119. [Crossref]
- Bruguera M, Miquel R (2007) The effect of hematological and lymphatic diseases on the liver. Textbook of Hepatology. 3rd ed. Oxford: Blackwell Publishing 1662-1670.
- El Badrawy A, Tawfiki AM, Mahmoud W, Abdel Salam E, Taalab MM et al (2016). Multidetector CT (MDCT) findings of primary hepatic lymphoma. Gulf J Oncolog 20: 64-70. [Crossref]
- Rajesh S, Bansal K, Sureka B, Patidar Y, Bihari C et al. (2015) The imaging conundrum of hepatic lymphoma revisited. Insights Imaging 6: 679-692. [Crossref]
- Lui YQ, Zhao FJ, Chen WQ (2009) Analysis of incidence and mortality of leukemia in China. China Cancer 22: 528-534.
- Bakst RL, Tallman MS, Douer D, Yahalom J (2011) How Treat extramedullary acute myeloid leukemia. Blood 118: 3785-3793. [Crossref]
- Cribe AS, Steenhof M, Marcher CW, et al. (2013) Extramedullary disease in patients with acute myeloid leukemia assessed by (18) F-FDG PET. Eur J Haematol 90: 273-278. [Crossref]
- Ciarallo A, Makis W, Novales Diaz JA, Michel RP (2011) Extramedullary gastric relapse of acute lymphoblastic leukemia following allergenic stem cell transplant: staging with F-18 FDG PET/CT. Clin Nucl Med 36: e90-e92. [Crossref]
- Wilputte JY, Martinet JP, Nguyen P, Damoiseaux P, Rahier J et al. (2003) Chronic lymphocytic leukemia with portal hypertension and without liver involvement: a case report underlining the roles of increased spleno-portal blood flow and “protective” sinusoidal vasoconstriction. Acta Gastroenterol Belg 66: 303-306. [Crossref]
