Mastication Problems and Dysphagia In 4 Patients with Treacher Collins Syndrome Due to Affected Orofacial Muscles
A B S T R A C T
Treacher Collins syndrome (TCS) is a rare congenital disorder of craniofacial development affecting 1 in 50 000 live births. The spectrum of the clinical features is wide ranging from mild to severe anatomical deviances, affecting breathing, mastication, swallowing, facial expression, hearing and speech. The aim of this study was to describe the experienced feeding and swallowing problems and to study the related orofacial muscles in four patients with Treacher Collins Syndrome (TCS), in order to increase important knowledge concerning mastication and swallowing problems in children with TCS.
Four children with TCS were evaluated with a clinical feeding and swallowing assessment. In addition, quantitative muscle ultrasound of orofacial muscles was performed.
The mastication problems and dysphagia are due to a range of problems. Orofacial muscles were hypoplastic with a deviant structure influencing strength. We recommend a regularly assessment with special attention to mastication, intake and growth. Training (maintain chewing) and compensation (adequate intake) should be advised.
Keywords
Treacher collins syndrome, dysphagia, mastication problems, muscle ultrasound, orofacial muscles
Introduction
Treacher Collins syndrome (TCS) is a rare congenital disorder of craniofacial development affecting 1 in 50 000 live births caused by mutations in TCOF1, or by POLR1D and POLR1C mutations in a smaller subset of TCS patients [1, 2]. The spectrum of the clinical features is wide ranging from mild to severe anatomical deviances, affecting breathing, mastication, swallowing, facial expression, hearing and speech [2, 3]. The deformities include defects in the periorbital region, hypoplasia of the mandible and zygomatic arches, and middle-ear deformities [1]. As a result of severe mandibular hypoplasia and airways narrowing, infants sometimes need a tracheostomy tube to establish breathing [1]. Previous studies on other craniofacial syndromes mentioned negative influences on the eating and drinking skills by choanal atresia and different forms of cleft palate [4]. Therefore, it is reasonable to assume that a combination of craniofacial abnormalities seems responsible for the feeding and swallowing difficulties in TCS [1]. Moreover, the feeding difficulties are supposed to be the cause of the often-reported thin posture in patients with TCS [3]. Wong et al. showed malar deformity on computed tomography (CT) in TCS and found a range of zygomatic hypoplasia with a decrease in malar volume compared with healthy participants [5]. Also, the masseter muscles were found to be thinner than in the control group. Furthermore, it was found that the reduced malar volume correlated with hypoplasia of the masseter muscles [6].
Plomp et al. (2016) provided in their systematic review current evidence for the multidisciplinary treatment in TCS. They provided a time schedule for respiratory and non-respiratory care for patients from 0 to 18 years. They found that some topics regarding treatment were well supported. However, items such as speech, feeding (and especially mastication) and swallowing are lacking sufficient evidence. To develop appropriate treatment options for feeding and swallowing problems it is important to study the underlying disturbed mechanisms in TCS. The aim of this study was to describe the experienced feeding and swallowing problems and to study the related orofacial muscles in four patients with TCS, in order to increase important knowledge concerning mastication and swallowing problems in children with TCS.
Material and Methods
All four patients experienced feeding or swallowing problems for which they were referred to our department of a university medical centre. They were evaluated with a clinical feeding and swallowing assessment by a speech language therapist (SLT). The Functional Oral Intake Scale (FOIS) and the Modified FOIS for young children were used to classify oral intake, ranging from 1 – nothing by mouth to 7 – total oral diet without any restrictions [7, 8]. Ultrasound proved to be a good technique for imaging muscle structure in terms of echogenicity and muscle thickness [9, 10]. As described quantitative muscle ultrasound (QMUS) uses gray scale analysis of the pictures to quantify muscle echogenicity and shows muscle thickness. Data are compared with normal values and described as z-scores. In the four patients we used the QMUS-technique on the submental muscles (digastric and geniohyoid muscles), the muscles for chewing (temporal and masseter muscle) and muscles of the tongue. In addition, thickness of the muscles and tongue were measured. In brief, muscle images were made with a broadband linear 10–5 MHz transducer using a Z.one convertible ultrasound system (Zonare Medical Systems; Mountain View, California), and were analyzed on thickness and echogenicity compared with normal values resulting in z-scores [9, 10]. In case of possible pharyngeal problems, a videofluroscopic swallow study (VFSS) was performed. The study was approved by the regional medical ethics committee (number 2018-4975, approved Jan 2019). Patients or their parents gave their written informed consent for publishing the data.
Table 1: Descriptive data
|
Patient |
Age (years; months) |
Gender |
Height; weight or BMI |
Hearing im-pairment |
Meatal atresia |
Choanal atresia |
Cleft palate |
Micro-gnathia |
Zygoma hypoplasia |
|
1 |
0;03 |
male |
60cm; 5.0kg* |
60dB CHI |
no |
no |
no |
mild |
yes |
|
2 |
2;04 |
female |
85cm; 11.0kg** |
60dB CHI |
yes |
bilateral |
no |
severe |
yes |
|
3 |
8;04 |
female |
BMI 16 |
60dB CHI |
yes |
bilateral |
no |
severe |
yes |
|
4 |
18;06 |
female |
BMI15 |
60dB CHI |
yes |
no |
yes (and cleft lip, right) |
severe |
yes |
CHI: conductive hearing impairment
* Height was between P16 and P50, weight was between P0.6 and P2
** Height was between P0.6 and P2, weight was at P16
Table 2: Data of the quantitative ultrasound of orofacial muscles. Data are depicted as z-scores, derived from healthy participants and corrected for age and height (9, 10).
|
|
m. digastricus left (z-score) |
m. digastricus right (z-score) |
m. genio-hyoideus* (z-score) |
m. masseter** (z-score) |
m. temporalis** (z-score) |
tongue*** (z-score) |
||||
|
|
T |
EG |
T |
EG |
EG |
T |
EG |
T |
EG |
T |
|
Patient 1 |
-0.4 |
0.2 |
nm |
nm |
-1.8 |
-2.5 |
-0.8 |
-2.7 |
3.7 |
-0.9 |
|
Patient 2 |
-2.0 |
3.3 |
-2.0 |
3.3 |
4.4 |
-3.9 |
7.9 |
nm |
nm |
-1.0 |
|
Patient 3 |
-2.2 |
-0.6 |
-2.0 |
-1.6 |
1.4 |
-3.2 |
8.1 |
-3.9 |
3.7 |
-1.5 |
|
Patient 4 |
-4.7 |
5.2 |
-3.8 |
4.8 |
11.5 |
-2.2 |
8.6 |
-4.8 |
6.5 |
-3.5 |
T= thickness; EG = echogenicity; nm = could not be measured
*the geniohyoid muscle left and right are measured together, because no clear boundary can be detected; **the masseter and temporal muscle were measured on the left side; ***in this study only thickness of the tongue was measured and no echogenicity data were collected.
Results
For all 4 patients the descriptive data are presented in (Table 1) and data of the QMUS of orofacial muscles are presented in (Table 2).
Patient 1
Patient 1 is a 0;03-year-old male with TCS (TCOF1 mutation, confirmed by genetic testing in the department of Human Genetics of our university medical centre) who presented with mild micrognathia and sucking and swallowing problems. In the neonatal period the SLT observed a disturbed coordination of sucking, swallowing and breathing, and in combination with the retracted tongue short apneas (4-6 seconds) were seen. To avoid these apneas the teat had to be removed out of his mouth several times during a feeding session. This breathing problem caused exhaustion with the need to offer supplementary nasogastric tube feeding. The feeding assessment turned to be level 3 on the Modified FOIS (meaning tube dependent with the consistent intake of fluids).
QMUS of the submental and mastication muscles was performed. Both the masseter and temporal muscle were found to be thin. Only the temporal muscle showed an increased echogenicity. Tube feeding was continued to prevent exhaustion during feeding.
Patient 2
Patient 2 is a 2;04 years old female with TCS (TCOF1 mutation, confirmed by genetic testing in the department of Human Genetics of our university medical centre) who received a tracheotomy tube for her acute airway obstruction at 2 days of age. Initially she received her feeding via a mouth tube. At 2 weeks of age oral feeding was started. Offering small amounts of milk (0.05ml) was combined with sucking on mother’s finger (finger feeding) to train sucking and swallowing. After a 4-weeks-training she was able to drink from a bottle. She needed a very soft nipple, because she lacked the strength to make a firm vacuum with the tongue. She was discharged from the hospital at 4 months of age. At the age of 9 months she was total orally fed and her parents started offering pureed fruit and vegetables with a spoon.
A follow-up assessment at the age of 28 months revealed a FOIS score of level 4.5 (Modified FOIS, meaning total oral diet, but requiring special preparation of solids). Her mealtime duration was prolonged and hard or tough solid food was difficult to chew. When she was tired her parents reported that consumption of bread crusts was impossible. All liquids were offered by a bottle. Cup drinking was yet to be started. The tracheotomy tube was still in place. QMUS of the submental and mastication muscles was performed and revealed a reduced thickness and increased echogenicity for all muscles.
Patient 3
Patient 3 is a 8;04 years old girl with TCS (TCOF1 mutation, confirmed by genetic testing in the department of Human Genetics of our university medical centre) who was referred to the SLT because of mastication difficulties. She needed supplementary tube feeding (gastrostomy) from birth on to 6 years of age. At time of the SLT assessment she had a tracheotomy tube with speaking valve and she was total orally fed. Her FOIS score was 5, because of prolonged mealtimes, the need for special preparations (no tough solid food) and compensations (cutting solid food in small pieces). Because she was able to chew parents judged her mastication problems as behavioral and were asking for an intensive program to speed up her eating of solid food. However, QMUS of the submental and mastication muscles showed very thin muscles with high echogenicity.
Patient 4
Patient 4 is an 18;06 years old female with TCS (POLRID deletion – TCS type 2, confirmed by genetic testing in the department of Human Genetics of our university medical centre) who just started her bachelor study after finishing high school. After birth, she was diagnosed with cleft palate and extreme micrognathia. She temporally needed a one-week- tracheotomy after surgery at respectively 1 and 11 years of age. At time of assessment by the SLT she complained about tiredness, losing weight and having not enough time to eat on schooldays. On the FOIS she had a score of level 6, because of difficulties with tough and hard solid food and raw food like salads. Mealtimes were prolonged.
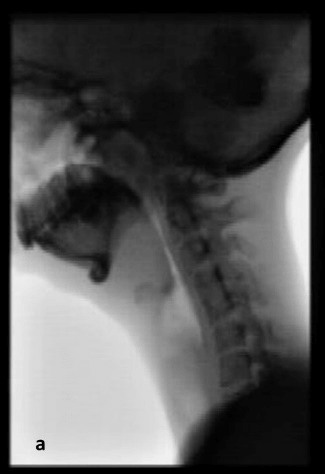
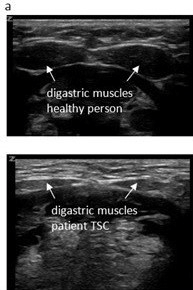
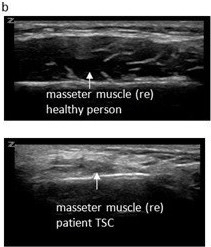
Observation of her mastication capacity and efficiency showed long lasting chewing with small chewing cycles, sometimes ending in spitting out the food that was not sufficient grinded. In addition, she complained of the sensation of food sticking in her throat. Therefore, a VFSS was performed with liquid and solid food. The VFSS revealed a prolonged oral phase, post swallow pharyngeal residue on solid food and a small nasopharyngeal area (Figure 1). QMUS of the submental and mastication muscles was performed and showed very thin digastric, masseter and temporal muscles, with a high echogenicity (Figure 2). Her tongue had a reduced thickness.
Figure 1: Images of the video fluoroscopic swallow study of patient 4 revealing pharyngeal post swallow residue on solid food (a) and a narrow pharyngeal area (b).
Figure 2: Ultrasound images of digastric muscles (a) and masseter muscles (b), upper part images of a healthy person (18 years), lower part images of patient 4
Discussion
All four patients with TCS experienced feeding problems and prolonged mealtimes. In addition, they all showed limited growth. They all showed the same kind of problems and QMUS of their orofacial muscles revealed the same trend. As stated by Plomp et al. (2016) evidence for the frequently oral facial dysfunction and feeding issues is scarce. This case series reveals new evidence for the affected orofacial muscles related to mastication and swallowing.
The masseter muscles were very thin which can be seen by the z-scores of < -2 for thickness in all four patients. This is in line with the findings of Wong et al. (2012) who used CT-scans to measure zygoma and masseter muscles. Masseter muscles were found to be significantly smaller than in healthy controls, and these authors posited that the disturbed embryologic development of the zygoma would affect the masseter muscle, due to a lack of biomechanical stimulation. However, this so called mechanical coupling is questionable, supposing bone and muscle cells interaction, both molecular and biomechanical during intrauterine development [11]. Nevertheless, this phenomenon might be an explanation that besides thinner mastication and tongue muscles, we also found a deviant structure of the masseter and temporal muscles expressed by the high z-scores on the echogenicity (> +2). A high echogenicity or whiter image means a disruption of normal architecture by which the muscle fibers are interspersed with fat and fibrosis [12]. Moreover, a high echogenicity of skeletal and mastication muscles reduces the ability to generate force (Jansen et al., 2012; van den Engel-Hoek et al., 2016).
The found reduced tongue thickness makes abnormal tongue movements likely and might explain both disturbed sucking movement (infants) and transport problems of chewable food to the molars. Similarly, it will influence chewing performance negatively. Furthermore, in some TCS patient’s salivary gland abnormalities might lead to a dry mouth, and diminished saliva will hamper mastication and swallowing even more [3]. It is plausible that the characteristic swallowing features (patient 1: problems with sucking, patient 2-4: prolonged mealtimes) could be explained by a combination of the above mentioned pathophysiological mechanisms.
Not only the mastication muscles were found to be affected, but we also detected involvement of the digastric and geniohyoid muscles in all patients (exception: the normal geniohyoid muscle in the youngest one). These so called submental muscles are playing an important role in the antero-superior hyoid movement during swallowing. This movement is responsible for the elevation and closing of the larynx with the epiglottis tipped over the closed vocal folds, and to open the upper esophageal sphincter to pass food to the esophagus. Previous study reported that affected submental muscles negatively influence the swallowing of especially thick liquid and solid food [13]. We confirmed this sticking from thick and solid foods by the VFSS in patient 4. After all, it became clear that her reduced oral intake and chewing performance had led to inadequate intake resulting in stress, losing weight and fatigue.
In feeding and swallowing rehabilitation the main aims are supporting sufficient growth and hydration, the development of feeding activities, ensuring safe swallowing and pleasant mealtimes for sufficient quality of life [14]. Rehabilitation must focus on training and compensation [15]. Based on our clinical experience in children and young adults with feeding and swallowing problems, and the found affected orofacial muscles in these four patients with TCS, we propose a regular feeding and swallowing assessment, in combination with the control of intake and growth. The treatment might include the advice to maintain chewing (‘use it or lose it’), in combination with easy consumable food, like high-caloric drinks. In addition, information for parents and children with TCS on the affected orofacial muscles and the consequences of it are important. As such, parental and patient misinterpretation were prevented in patient 3 and 4, and diet adjustments avoided them from ending up in a downward spiral.
Conclusion
The mastication problems and dysphagia in patients with TCS are due to a range of problems. Quantitative muscle ultrasound is an easy to perform and non-invasive method to assess orofacial muscles. It gives valuable information on affected muscles in order to understand experienced problems. With this case series we have shown that orofacial muscles in these patients are hypoplastic and have a deviant structure, influencing strength. A regularly feeding and swallowing assessment in patients with TCS is recommended, with special attention to mastication problems, intake and growth. Training (maintain chewing) and compensation (adequate intake) should be advised.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Abbreviations
Treacher Collins syndrome (TCS)
computed tomography (CT)
Functional Oral Intake Scale (FOIS)
quantitative muscle ultrasound (QMUS)
video fluoroscopic swallow study (VFSS)
Article Info
Article Type
Research ArticlePublication history
Received: Thu 01, Aug 2019Accepted: Fri 16, Aug 2019
Published: Mon 30, Sep 2019
Copyright
© 2023 Lenie van den Engel-Hoek. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2019.03.09
Author Info
C.E. Erasmus L. van Haaften Lenie van den Engel-Hoek M. Lagarde M. van Gerven R.J.C. Admiraal
Corresponding Author
Lenie van den Engel-HoekDepartment of Rehabilitation, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behavior, Nijmegen, the Netherlands
Figures & Tables
Table 1: Descriptive data
|
Patient |
Age (years; months) |
Gender |
Height; weight or BMI |
Hearing im-pairment |
Meatal atresia |
Choanal atresia |
Cleft palate |
Micro-gnathia |
Zygoma hypoplasia |
|
1 |
0;03 |
male |
60cm; 5.0kg* |
60dB CHI |
no |
no |
no |
mild |
yes |
|
2 |
2;04 |
female |
85cm; 11.0kg** |
60dB CHI |
yes |
bilateral |
no |
severe |
yes |
|
3 |
8;04 |
female |
BMI 16 |
60dB CHI |
yes |
bilateral |
no |
severe |
yes |
|
4 |
18;06 |
female |
BMI15 |
60dB CHI |
yes |
no |
yes (and cleft lip, right) |
severe |
yes |
CHI: conductive hearing impairment
* Height was between P16 and P50, weight was between P0.6 and P2
** Height was between P0.6 and P2, weight was at P16
Table 2: Data of the quantitative ultrasound of orofacial muscles. Data are depicted as z-scores, derived from healthy participants and corrected for age and height (9, 10).
|
|
m. digastricus left (z-score) |
m. digastricus right (z-score) |
m. genio-hyoideus* (z-score) |
m. masseter** (z-score) |
m. temporalis** (z-score) |
tongue*** (z-score) |
||||
|
|
T |
EG |
T |
EG |
EG |
T |
EG |
T |
EG |
T |
|
Patient 1 |
-0.4 |
0.2 |
nm |
nm |
-1.8 |
-2.5 |
-0.8 |
-2.7 |
3.7 |
-0.9 |
|
Patient 2 |
-2.0 |
3.3 |
-2.0 |
3.3 |
4.4 |
-3.9 |
7.9 |
nm |
nm |
-1.0 |
|
Patient 3 |
-2.2 |
-0.6 |
-2.0 |
-1.6 |
1.4 |
-3.2 |
8.1 |
-3.9 |
3.7 |
-1.5 |
|
Patient 4 |
-4.7 |
5.2 |
-3.8 |
4.8 |
11.5 |
-2.2 |
8.6 |
-4.8 |
6.5 |
-3.5 |
T= thickness; EG = echogenicity; nm = could not be measured
*the geniohyoid muscle left and right are measured together, because no clear boundary can be detected; **the masseter and temporal muscle were measured on the left side; ***in this study only thickness of the tongue was measured and no echogenicity data were collected.

References
- Plomp RG, van Lieshout MJ, Joosten KF, Wolvius EB, van der Schroeff MP et al. (2016) Treacher Collins Syndrome: A Systematic Review of Evidence-Based Treatment and Recommendations. Plast Reconstr Surg 137: 191-204. [Crossref]
- van Gijn DR, Tucker AS, Cobourne MT (2013) Craniofacial development: current concepts in the molecular basis of Treacher Collins syndrome. Br J Oral Maxillofac Surg 51: 384-388. [Crossref]
- Asten P, Skogedal N, Nordgarden H, Axelsson S, Akre H (2013) Orofacial functions and oral health associated with Treacher Collins syndrome. Acta Odontol Scand 71: 616-625. [Crossref]
- Cooper-Brown L, Copeland S, Dailey S, Downey D, Petersen MC et al. (2008) Feeding and swallowing dysfunction in genetic syndromes. Dev Disabil Res Rev 14: 147-157. [Crossref]
- Wong KR, Pfaff MJ, Chang CC, Travieso R, Steinbacher DM (2013) A range of malar and masseteric hypoplasia exists in Treacher Collins syndrome. J Plast Reconstr Aesthet Surg 66: 43-46. [Crossref]
- Magalhaes MH, da Silveira CB, Moreira CR, Cavalcanti MG (2007) Clinical and imaging correlations of Treacher Collins syndrome: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol 103: 836-842. [Crossref]
- Crary MA, Mann GD, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86: 1516-1520. [Crossref]
- Dodrill P, Gosa MM (2015) Pediatric Dysphagia: Physiology, Assessment, and Management. Ann Nutr Metab 5: 24-31. [Crossref]
- van den Engel-Hoek L, van Alfen N, de Swart BJ, de Groot IJ, Pillen S (2012) Quantitative ultrasound of the tongue and submental muscles in children and young adults. Muscle Nerve 46: 31-37. [Crossref]
- Lagarde MLJ, van den Engel-Hoek L (2017) Quantitative ultrasound of orofacial muscles in infants between 6 months and 5 years. Curr Med Imag Rev 13: 332-338.
- Brotto M, Bonewald L (2015) Bone and muscle: Interactions beyond mechanical. Bone 80: 109-114. [Crossref]
- van Den Engel-Hoek L, Lagarde M, Van Alfen N (2017) Ultrasound of oral and masticatory muscles: Why every neuromuscular swallow team should have an ultrasound machine. Clin Anat 30: 183-193. [Crossref]
- van den Engel-Hoek L, Erasmus CE, Hendriks JC, Geurts AC, Klein WM et al. (2013) Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment. J Neurol 260: 1295-1303. [Crossref]
- van den Engel-Hoek L, Harding C, van Gerven M, Cockerill H (2017) Pediatric feeding and swallowing rehabilitation: An overview. J Pediatr Rehabil Med 10: 95-105. [Crossref]
- Ramdharry GM (2010) Rehabilitation in practice: management of lower motor neuron weakness. Clin Rehabil 24: 387-397. [Crossref]
