Management and Outcomes of Spontaneous Rupture of Hepatocellular Carcinoma: Current Practice
A B S T R A C T
Objectives: To review the current management options for ruptured hepatocellular carcinoma (HCC) at acute presentation and assess the impact of each treatment modality on short- and long-term patient related outcome.
Study design: A PubMed search was undertaken for review articles from 1950 to 2019 using key phrases “Ruptured hepatocellular carcinoma”, “trans-arterial embolization”, “resection”, “computed tomographic scan” and “conservative management”. Further manual search was performed to identify key articles from the reference list.
Methodology: All papers with previous described management for ruptured HCC were reviewed. The morbidity, mortality and comparison of various management options were reviewed. Current practice guidelines were visited to identify common practice protocols.
Conclusion: Ruptured HCC is associated with significant morbidity and mortality. Multiple management options can be applied guiding by patient’s overall condition. Conservative management is associated with overall poor outcome. Staged liver resection is associated with better outcome with improved morbidity and mortality.
Keywords
Ruptured hepatocellular carcinoma, trans-arterial embolization, resection, computed tomographic scan
Introduction
Hepatocellular carcinoma (HCC) has been a major contributor to health-related disorders in the developed and developing world [1]. Approximately 0.7 million deaths are attributed to HCC [2]. Both hepatitis B and C are major contributors to chronic hepatitis leading up to cirrhosis. 80% of HCC develops in cirrhotic liver [3-5]. Spontaneous rupture is an uncommon presentation of HCC. There is considerable geographical variation in presentation worldwide with reported incidence in western world (<5%) to a significantly high presentations in Asia (2.9% - 14%) [3, 6]. The incidence of ruptured HCC is decreasing since more patients are undergoing diagnostic and surveillance imaging for cirrhosis [6]. The acute rupture episode is associated with significant morbidity and mortality (up to 75%) [1]. The increased mortality is related to episode of acute liver failure (40%) following rupture [7]. There is considerable variation in presentation of ruptured HCC from asymptomatic to haemorrhagic shock. Most patients with acute rupture have an underlying decompensated liver disease which precipitates coagulopathy, multi-organ failure [3]. The options for treatment of ruptured HCC are limited. Conservative management is associated with poor outcome with mortality reaching up to 100% [8]. Once patient is initially stabilised and haemorrhagic shock has been addressed focus of management should shift to options available for management of ruptured HCC. Each case has to be individualised based on patient condition and tumour stage. A multidisciplinary approach initiated which includes urgent laparotomy and peri-hepatic packing for haemorrhage control, emergency liver resection or trans-arterial embolisation (TAE) [9]. The aim of this study is to analyse the presentation, management, short- and long-term outcome of patients managed with ruptured HCC. We also aim to review the treatment strategy adopted and changes in management of ruptured HCC (surgical versus nonsurgical) with the introduction of interventional radiology in routine clinical practice.
Pathogenesis of rupture
The exact mechanism of tumour rupture is poorly understood. Multiple theories have been put forward, but no consensus has been reached. Both tumour related hepatic invasion, extra-hepatic spread, tumour size and underlying liver characteristics of cirrhosis and portal vein thrombus have all been implicated as risk factors for spontaneous rupture [10].Tumour locations in segment II, III, VI, VII were identified as common site for occurrence of ruptured HCC. This is based on theory that these segments of the liver have less volume and are located in the periphery. When tumours grow in these segments with less liver volume, they become exophytic surrounded by very thin rim of normal liver parenchyma. Soon as the outer wall of tumour becomes thin and erodes into the liver capsule it can spontaneously rupture causing catastrophic intra-peritoneal haemorrhage [11].
The obstruction in hepatic venous outflow either with direct tumour invasion or tumour thrombus is a potential risk factor in the development of intra-parenchymal tumour related haemorrhage. Since tumour venous outflow drains towards hepatic veins and any retrograde pressure can significantly disrupt the fragile tumour vasculature which has significant collagen type IV degradation [12]. There are significant changes identified in tumour vessel walls with aggressive growth of the tumour cells and increased expression of pro-angiogenic factors resulting in disorganised vasculature. Vessels become tortuous and have increased permeability. This explains why even small HCC < 2 cm can also spontaneously rupture [13]. Tumour size of more than 5 cm is also associated with high risk of rupture [10]. Previously treated HCC with tran-arterial chemo-embolisation (TACE) and selective internal radiation therapy (SIRT) can also pre-dispose to spontaneous haemorrhage [14]. This is attributed to acute necrotic event within the tumour and associated damage to vessel wall causing spontaneous disruption of vessel integrity leading to intra-parenchymal haemorrhage. Even abdominal trauma and coagulopathy associated with underlying liver failure are also described as causative factors for spontaneous rupture of HCC [5]. Hepatic lesions that rupture spontaneously can both be intrahepatic or exophytic.
Clinical Presentation
Ruptured HCC is a spontaneous event with no significant pre-event features. Most common presentation is with sudden onset of abdominal pain (55-100%) and shock (30-905%) [14]. Up to 40% of patients also develop acute liver failure at time of presentation with hepatic encephalopathy and confusion [1]. It is interesting to note that most patients with ruptured HCC are not identified to be in terminal stage of disease or do not have disseminated cancer.
Diagnosis
High index of clinical suspicion has to be maintained in prompt diagnosis of these patients. Emergency department (ED) diagnostic abdominal paracentesis can be performed to assess for haemo-peritoneum. However negative aspirate should not preclude from performing advanced diagnostic abdominal imaging. Ultrasound performed in the ED can identify fluid in the peritoneal cavity and capsular disruption of the liver but has decreased overall sensitivity and specificity. Immediate triphasic Computed tomography (CT) can identify ruptured HCC in up to 75% of cases (Figure 1). Multiple CT features have been described in literature which help to confirm and identify the site of the haemorrhage. On arterial phase imaging active contrast extravasation can be identified and along with capsular disruption is a significant determinant of a spontaneous rupture of HCC [15].
Figure 1: Rupture of segment 4b exophytic tumour with haemoperitoneum.
I Acute phase management of ruptured HCC
The primary objective in immediate management of ruptured HCC is haemorrhage control. Several treatment strategies have been employed including conservative management, trans-arterial embolization (TAE), surgical packing, liver resection and two staged hepatectomy [16]. There is no current consensus on optimal approach in the management of ruptured HCC. These patients present with hypovolemic shock, disseminated intra-vascular coagulation, liver failure and multi-organ failure. Most patients will require initial intensive care management to achieve manage multi-organ failure. During 1950’s to 1980’s the main stay of management was exploratory laparotomy leading to peri-hepatic packing, suture plication, hepatic artery ligation, alcohol injection and formal liver resection were employed. All these procedures to achieve haemostasis were associated with significant morbidity and mortality. Table summarises studies published in the past summarising various methods employed and their one- and 3-year survival outcomes.
II Conservative management
Non-operative management can be employed in patients with stable haemodynamic status. These patients will be admitted to the intensive care settings. Rapid correction of coagulopathy, acid base status and renal replacement therapy according to clinical indications will be initiated. In previous case series most patients with advanced age and inoperable tumours were treated with non-operative management. This approach was un-surprisingly associated with significant mortality [17]. Patient’s liver function tests and liver failure parameters are continuously monitored, and any signs of acute decompensations are immediately addressed. If there are signs of ongoing bleeding, patients with haemodynamic stability can proceed to TAE. For patients who become haemodynamically unstable, surgery becomes a necessity.
Figure 2: Trans-arterial embolisation of segment 8 intra-parenchymal rupture of hepatocellular carcinoma.
III Interventional radiology tran-arterial embolisation and radiofrequency ablation
TAE is considered first line in achieving haemostasis with a success rate of 75% to 100% (Figure 2) [18]. Following TAE, recurrent bleeding and post-embolisation liver failure can result in increased in-hospital mortality (up to 50%) [19]. Portal vein thrombosis is a relative contraindication for TAE. However super selective embolization can still be performed without risk of infraction of full liver [20]. Bilirubin > 50µmol/L at time of diagnosis have shown poor outcome following emergency TAE [7, 20]. TAE (0-37%) has significantly reduced 30 days mortality versus surgery (28-75%) [1]. Long term outcome of TAE only management is still poor [22, 23]. Therefore, to achieve long term survival these patients have to be considered for surgical resection on individual merit. Following initial haemostatic control with TAE, trans-arterial catheter chemo-embolisation (TACE) is performed for non-surgical management or downsizing of tumour for potential resection [7]. There is considerable published evidence to support surgery followed by TACE in setting of ruptured HCC [19, 24-27]. Radiofrequency ablation (RFA) has been employed in acute settings to achieve haemostasis with limited success [28, 29].
Surgical Management
I Peri-hepatic packing
There is considerable lack of data on peri-oprative packing in ruptured HCC. Most of the experience and outcomes are derived from liver trauma patients. It is described as an additional adjunct strategy to consider in patients with significant haemorrhage and require immediate stabilisation during damage control surgery [1]. Peri-hepatic packing left in situ for more than 48 is associated with significant risk of abscess (23%) and sepsis (32%) [14]. Also, the removal of packing pre-disposes to re-bleeding.
II Suture plication
Small bleeding sites with well-preserved liver parenchyma are suitable for suture plication but require exploratory laparotomy. There are no large case series in the literature of the success of this strategy [1]. Both 2/0 prolene and chromic suture materials have been employed with varied success. 26.9% survival has been described in a small case series with a combination of plication and packing techniques were employed [14].
III Hepatic artery ligation
Liver is perfused by both portal vein (70%) and hepatic artery (30%). However, HCC depends exclusively on hepatic artery. Intra-operative ligation of hepatic artery achieves haemostasis (70 -100%) but associated with increased post-procedure mortality (60-70%) [30]. Selective ligation of hepatic artery can help for a subsequent TACE or liver resection procedure with less co-morbidity. The effect from ligation is temporary since the tumour will develop collateral arterial supply over following weeks. Hence a management plan should be individualised in early post-arterial ligation phase.
IV Liver resection
Formal liver resection in acute phase is associated with considerable mortality (16-100%) and is only recommended in patients with acute haemodynamic instability [1, 31]. Several published studies with small case series have reported outcomes of liver resections in patients with acute ruptured HCC. In these patients’ lesions were peripherally located and liver function was well preserved (Child Pugh Class A or B) [32, 33]. The rationale for early surgical resection is to prevent tumour invasion of the venous system which will lead to systemic spread. However, staged liver resection following initial stabilisation is the preferred approach with significant reduced mortality (9%) and improved one-year survival rate (55-100%) [1]. Patient will complete all pre-operative staging work-up and evaluation before surgical resection. This delay helps improving patient fitness and more time to achieve nutritional balance. There is no defined time range for resection and is guided by individual centre experience and exposure. Also, laparoscopic liver resection has also been performed for ruptured HCC [34]. Delay in surgery in patients suitable for resection achieves better outcome. There are only few case reports of liver transplantation following ruptured HCC. In most liver transplant allocation systems ruptured HCC is a contraindication for listing [35].
Survival and long-term outcome following HCC rupture
Serum bilirubin levels, pre-morbid disease condition and shock at presentation are important determinants of overall outcome of patients presenting with ruptured HCC [1]. Survival following conservative management is generally poor with 0% reported survival at one year [36]. The BCLC staging has been employed to assess the survival in ruptured HCC patients with stage A (251 days), B (175 days) and C (40 days). One-year outcome is significantly better with TACE (28.6%) and emergency liver resection (59.4%) [36]. Also staged liver resection has reportedly significantly improved survival to both TACE and early liver resection (54.2-100%) [1].
Table 1: Previous published case series with one- and three-years outcomes in patients treated with ruptured HCC.
|
Sample size |
Management |
In-hospital /30-day Mortality |
One- Year Survival |
3-year survival |
|
|
Leung (37) 2002 |
31 |
TAE |
26% |
NA |
NA |
|
Vergara (38) 2000 |
6 |
Emergency hepatectomy |
16.5% |
NA |
50% |
|
Yeh (39) 2002 |
60 |
Staged liver resection |
7.1 |
54.2% |
35% |
|
Mizuno (40) 2004 |
6 |
TAE followed by resection |
NA |
69.3% |
21.2% |
|
Hsueh (41) 2012 |
54 |
Non-surgical 6 (11.1%) TAE only 29(53.7%) Emergency Liver resection 19 (41.3%) Staged liver resection 18(33.3%) |
NA 18.2% NA 2.7% |
NA 18% NA 62.2% |
NA NA NA NA |
|
Aoki (42) 2013 |
1106 |
Non-surgical 275 (24.9%) TAE only 489 (44.2%) Liver resection 298 (26.9%) |
NA NA NA |
NA 39.7% 76% |
NA 14.1% 48.6% |
Trans-arterial embolization, NA: Not available
Conclusion
One of the important factors in Western world for low incidence of ruptured HCC as compared to Eastern population is continuous imaging surveillance programs in high risk population e.g. Cirrhosis. Therefore, most hepatic lesions are picked early and treated. Most of the literature from ruptured HCC is based on retrospective case series from Far East. All decisions and options in management of such patients has to be individualised within the setup of a multi-disciplinary team. Spontaneous rupture of HCC is generally a life-threatening emergency and early recognition and imaging can help to improve outcome. TAE followed by planned liver resection is the procedure of choice. But if the patient is in acute shock then an emergency laparotomy to achieve haemostatic control is the acceptable management option. A combination of TAE and staged liver resection provide best outcome; however, the overall survival and prognosis is poor.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 05, Nov 2019Accepted: Tue 19, Nov 2019
Published: Thu 28, Nov 2019
Copyright
© 2023 Ahmad Mirza. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.06.04
Author Info
Ahmad Mirza Arslan Pannu Eloise Lawrence Ghulam Murtaza Dar Khurram Khan Salman Jabbar Shahzad Ahmed
Corresponding Author
Ahmad MirzaDepartment of Abdominal Transplant and Hepato-Biliary Surgery, University of Cincinnati Medical Center, Ohio, USA
Figures & Tables
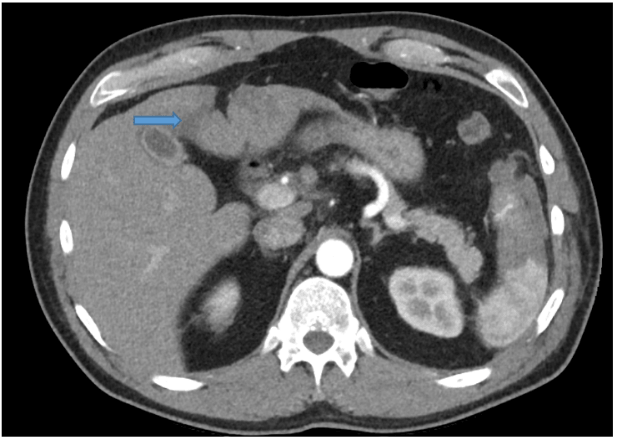
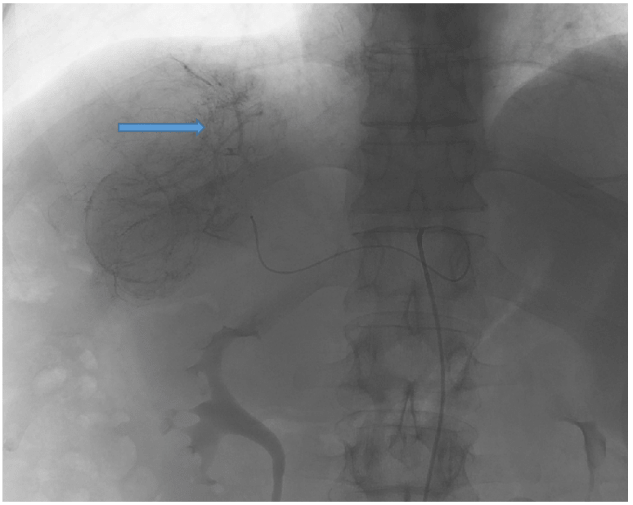
Table 1: Previous published case series with one- and three-years outcomes in patients treated with ruptured HCC.
|
Sample size |
Management |
In-hospital /30-day Mortality |
One- Year Survival |
3-year survival |
|
|
Leung (37) 2002 |
31 |
TAE |
26% |
NA |
NA |
|
Vergara (38) 2000 |
6 |
Emergency hepatectomy |
16.5% |
NA |
50% |
|
Yeh (39) 2002 |
60 |
Staged liver resection |
7.1 |
54.2% |
35% |
|
Mizuno (40) 2004 |
6 |
TAE followed by resection |
NA |
69.3% |
21.2% |
|
Hsueh (41) 2012 |
54 |
Non-surgical 6 (11.1%) TAE only 29(53.7%) Emergency Liver resection 19 (41.3%) Staged liver resection 18(33.3%) |
NA 18.2% NA 2.7% |
NA 18% NA 62.2% |
NA NA NA NA |
|
Aoki (42) 2013 |
1106 |
Non-surgical 275 (24.9%) TAE only 489 (44.2%) Liver resection 298 (26.9%) |
NA NA NA |
NA 39.7% 76% |
NA 14.1% 48.6% |
Trans-arterial embolization, NA: Not available
References
- Lai EC, Lau WY (2006) Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg 141: 191-198. [Crossref]
- J F, HR S, F B, D F, Mathers C et al. (2010) Esimate of Worldwide burden of cancer in 2008. Globocan Inj Cancer 127: 2893-2917. [Crossref]
- Letchumanan VP, Lim KF, Mohamad AB (2013) Diagnosis and management of ruptured hepatoma: single center experience over 10 years. Med J Malaysia 68: 405-409. [Crossref]
- Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127: S5-S16. [Crossref]
- Lau WY (2002) Management of hepatocellular carcinoma. J R Coll Surg Edinb 47: 389-399. [Crossref]
- Zhang H, Cong J, Chen C (2007) Spontaneous rupture of primary hepatocellular carcinoma: Experience of Emergency laparotomy over a 16-year period. Chinese J Clin Oncol 4: 322-326.
- CS L, CN T, KH F, MK L (2002) A retrospective review of transcatheter hepatic arterial embolisation for ruptured hepatocellular carcinoma. J R Coll Surg Edinb 47: 685-688. [Crossref]
- Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, Boonyapisit S (1983) Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer 51: 1532-1536. [Crossref]
- Jin YJ, Lee JW, Park SW, Lee JI, Lee DH et al. (2013) Survival outcome of patients with spontaneously ruptured hepatocellular carcinoma treated surgically or by transarterial embolization. World J Gastroenterol 19: 4537-4544. [Crossref]
- Zhu Q, Li J, Yan JJ, Huang L, Wu MC et al. (2012) Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol 18: 7302-7307. [Crossref]
- Li J, Huang L, Liu CF, Cao J, Yan JJ et al. (2014) Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol 20: 9121-9127. [Crossref]
- Lx Z (1996) Spontaneous rupture of hepatocellular carcinoma Br J Surg 83: 602-607.
- Tanaka A, Takeda R, Mukaihara S, Hayakawa K, Shibata T et al. (2001) Treatment of ruptured hepatocellular carcinoma. Int J Clin Onco 6: 291-295. [Crossref]
- Miyamoto M, Sudo T, Kuyama T (1991) Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol 86: 67-71. [Crossref]
- K M, T J, V L (2007) Findings of ruptured hepatocellular carcinoma on computed tomography in Thailand. Asian Biomed 1: 281-284. [Crossref]
- Yang H, Chen K, Wei Y, Liu F, Li H et al. (2014) Treatment of spontaneous ruptured hepatocellular carcinoma: A single-center study. Pak J Med Sci 30: 472-476. [Crossref]
- Ong GB, Chu EP, Yu FY, Lee TC (1965) Spontaneous Rupture of Hepatocellular Carcinoma. Br J Surg 52: 123-129.
- Lubner M, Menias C, Rucker C, Bhalla S, Peterson CM et al. (2007) Blood in the belly: CT findings of hemoperitoneum. Radiographics 27: 109-125. [Crossref]
- Li WH, Cheuk EC, Kowk PC, Cheung MT (2009) Survival after transarterial embolization for spontaneous ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 16: 508-512. [Crossref]
- Yoshida H, Mamada Y, Taniai N, Uchida E (2016) Spontaneous ruptured hepatocellular carcinoma. Hepatol Res 46: 13-21. [Crossref]
- Kirikoshi H, Saito S, Yoneda M, Fujita K, Mawatari H et al. (2009) Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol 9: 29. [Crossref]
- Kim JY, Lee JS, Oh DH, Yim YH, Lee HK (2012) Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol 24: 640-645. [Crossref]
- Wang B, Lu Y, Zhang XF, Yu L, Pan CE et al. (2008) Management of spontaneous rupture of hepatocellular carcinoma. ANZ J Surg 78: 501-503.
- Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI et al. (2006) Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg 10: 379-386. [Crossref]
- Maoz D, Sharon E, Chen Y, Grief F (2010) Spontaneous hepatic rupture: 13-year experience of a single center. Eur J Gastroenterol Hepatol 22: 997-1000. [Crossref]
- Tarantino L, Sordelli I, Calise F, Ripa C, Perrotta M (2011) Prognosis of patients with spontaneous rupture of hepatocellular carcinoma in cirrhosis. Updates Surg 63: 25-30. [Crossref]
- Marini P, Vilgrain V, Belghiti J (2002) Management of spontaneous rupture of liver tumours. Dig Surg 19: 109-113. [Crossref]
- Bertacco A, D’Amico F, Romano M, Finotti M, Vitale A et al. (2017) Liver radiofrequency ablation as emergency treatment for a ruptured hepatocellular carcinoma: a case report. J Med Case Rep 11: 54. [Crossref]
- Cheung TT, Poon RT, Chok KS, Chan AC, Tsang SH et al. (2014) Management of spontaneously ruptured hepatocellular carcinomas in the radiofrequency ablation era. PLoS One 9: e94453. [Crossref]
- Dewar GA, Griffin SM, Ku KW, Lau WY, Li AK (1991) Management of bleeding liver tumours in Hong Kong. Br J Surg 78: 463-466. [Crossref]
- Yoshida H, Onda M, Tajiri T, Umehara M, Mamada Y et al. (1999) Treatment of spontaneous ruptured hepatocellular carcinoma. Hepatogastroenterology 46: 2451-2453. [Crossref]
- Battula N, Madanur M, Priest O, Srinivasan P, O'Grady J et al. (2009) Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg 197: 164-167. [Crossref]
- Hai L, Yong-Hong P, Yong F, Ren-Feng L (2005) One-stage liver resection for spontaneous rupture of hepatocellular carcinoma. World J Surg 29: 1316-1318. [Crossref]
- Belgaumkar A, Carswell KA, Patel AG (2009) Laparoscopic resection of ruptured liver tumors. J Laparoendosc Adv Surg Tech A 19: 641-645. [Crossref]
- Blood NHS (2009) Transplant liver advisory group Protocols and guidelines for adults undergoing deceased donor liver transplantation in the UK.
- Zhong F, Cheng XS, He K, Sun SB, Zhou J et al. (2016) Treatment outcomes of spontaneous rupture of hepatocellular carcinoma with hemorrhagic shock: a multicenter study. Springerplus 5: 1101. [Crossref]
- Leung CS, Tang CN, Fung KH, Li MK (2002) A retrospective review of transcatheter hepatic arterial embolisation for ruptured hepatocellular carcinoma. J R Coll Surg Edinb 47: 685-688. [Crossref]
- Vergara V, Muratore A, Bouzari H, Polastri R, Ferrero A et al. (2000) Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol 26: 770-772. [Crossref]
- Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC (2002) Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg 89: 1125-1129. [Crossref]
- Mizuno S, Yamagiwa K, Ogawa T, Tabata M, Yokoi H et al. (2004) Are the results of surgical treatment of hepatocellular carcinoma poor if the tumor has spontaneously ruptured? Scand J Gastroenterol 39: 567-570. [Crossref]
- Hsueh KC, Fan HL, Chen TW, Chan DC, Yu JC et al. (2012) Management of spontaneously ruptured hepatocellular carcinoma and hemoperitoneum manifested as acute abdomen in the emergency room. World J Surg 36: 2670-2676. [Crossref]
- Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T et al. (2014) Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg 259: 532-542. [Crossref]
