Malignant Ovarian Dysgerminoma Following Ovulation Induction in Patients with Polycystic Ovarian Syndrome: A Literature Review and Two Case Reports
A B S T R A C T
Background: The relation of ovarian induction drugs to development of ovarian cancer is still unclear. Polycystic ovary syndrome (PCOS) is a complex endocrine disorder that has been reported as a risk factor for ovarian cancer in some studies.
Case presentation: We report two cases with polycystic ovarian syndrome who developed a malignant ovarian dysgerminoma following use of ovulation induction drugs. One of them was diagnosed during fifth month of pregnancy by intracytoplasmic injection (ICSI). Both cases were treated with conservative surgery.
Conclusion: We reported 2 cases of ovarian dysgerminoma in patients with PCOS following ovarian stimulation. A large multicenter observational study is recommended to investigate a possible association.
Keywords
Polycystic ovarian syndrome, ovulation induction, ovarian cancer, dysgerminoma
Background
There is increasing use of fertility medications for ovulation induction and ovarian stimulation for in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in the treatment of female infertility. The relation of assisted reproduction to development of ovarian cancer is still unclear. It is well-known that uninterrupted ovulation causing repeated ovarian epithelial surface damage and repair underlies the ‘incessant ovulation theory’ [1]. Use of fertility medications could increase ovarian cancer risk by promoting polyfollicular ovulation and ovulation trauma [1]. An alternate mechanism underlying the relationship between fertility treatment and ovarian cancer is the ‘elevated gonadotropin’ theory, which postulates that elevated gonadotropin levels resulting from fertility medications stimulate the ovarian epithelium and may induce malignant changes [2]. Two studies found an association between fertility medication use and ovarian cancer [3, 4]. While most recent studies and systematic reviews show no overall association between fertility medication use and invasive ovarian cancer when using a subfertile control group or the general population [5].
Furthermore, in a Cochrane database systematic review, Rizzuto et al. found no convincing evidence of an increase in the risk of invasive ovarian tumors with fertility drug treatment but there may be an increased risk of borderline ovarian tumors in subfertile women treated with in-vitro fertilization (IVF) [6]. The review was updated in 2019 and concluded that some studies added a supporting evidence to suggest that infertility drugs may increase the risk of ovarian cancer slightly in subfertile women treated with infertility drugs when compared to the general population or to subfertile women not treated. The risk is slightly higher in nulliparous than in multiparous women treated with infertility drugs [7]. Polycystic ovary syndrome (PCOS) is a complex endocrine disorder characterized by oligo-ovulation, hyperandrogenism, and certain ultrasound criteria [8]. PCOS has been reported as a risk factor for ovarian cancer in multiple studies [9, 10]. However, some studies have reported a reduced risk of ovarian cancer among women with oligomenorrhea [1, 10].
We report two cases with polycystic ovarian syndrome who developed a malignant ovarian dysgerminoma following ovulation induction. One of them was diagnosed during fifth month of pregnancy by ICSI. To the best of our knowledge; this association was not described before.
Case Presentations
Case I
A 23 years old female complained of primary infertility for 5 years because of polycystic ovarian syndrome (PCOS). Her husband had normal semen analysis and other fertility factors were normal. The couple decided to start an assisted reproduction trial on June 2018. Prior to the trial; the ovaries were seen by transvaginal ultrasound with polycystic criteria with no abnormal cystic or solid masses. To avoid ovarian hyperstimulation; the ovaries were stimulated using an antagonist protocol and triggering was done using LHRH analogue (Decapeptyl 0.1 mg ampoules subcutaneous injection). Ovum pick up was performed on 03/07/ 2018 and during oocyte retrieval ovaries were normal with no solid masses. Forty oocytes were retrieved, 32 were M2 grade that were fertilized by intracytoplasmic sperm injection (ICSI) technique resulted in 24 embryos of grade A. All embryos were cryopreserved at day-3 by verification. After thawing, 2 good-quality embryos were transferred to uterus 2 months later (on 01/09/2018).
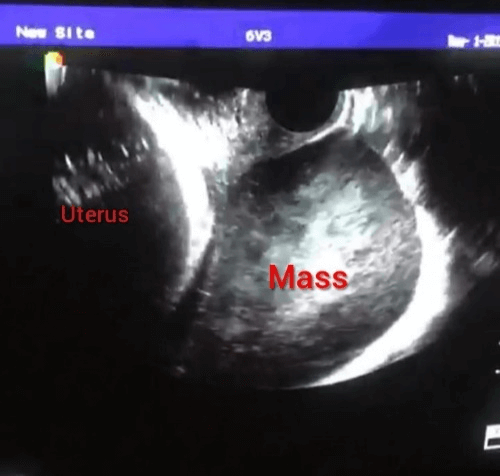
Pregnancy was confirmed and continued with one normal fetus. The course of pregnancy was normal till the twenty-second week where a solid right ovarian mass 5X5 cm accidentally discovered during routine ultrasound scanning with no ascites and other abdominal organs were normal (Figure 1). Lactate dehydrogenase was found to be high (2400 mg/ml) raising the possibility of germ cell tumors of the ovary. The patient and her husband were informed about the condition and counseled about options of treatment and prognosis, but they chose to continue pregnancy till fetal maturity.
Figure 1: Transvaginal ultrasound picture of case 1 at 22 weeks of pregnancy showing a solid ovarian mass on one side of the gravid uterus.
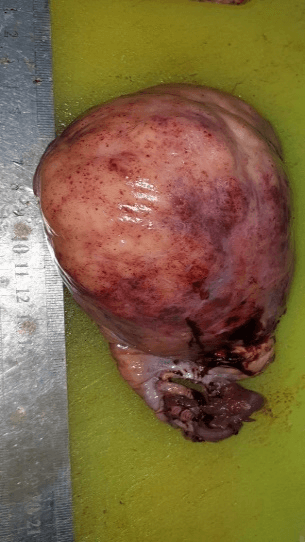
At completed 37 weeks, an elective caesarean section was performed during which a staging laparotomy was done. The tumor was confined to right ovary with intact capsule measuring 11X9 cm (Figure 1). Liver, omentum, and intestine were free. No iliac or para-aortic lymph node enlargement. The neonate was a healthy female. A fertility-sparing right salpingo-oophorectomy with omental biopsy was performed (Figure 2).
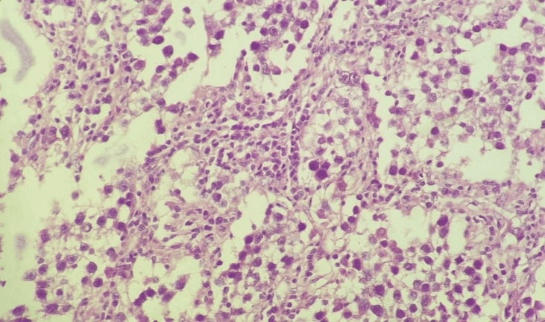
Post-operative histopathology revealed ovarian dysgerminoma with focal infiltration of the capsule (Figure 3) with negative peritoneal cytology and free omental biopsy (stage Ic2). Immunohistochemical staining with CD 117 monoclonal antibody revealed diffuse positive membranous and cytoplasmic reaction (Figure 4). According to tumor board recommendations the patient was advised to receive 4 courses of BEP (Bleomycine, Etopozide, Cisplatinum) chemotherapy as an adjuvant treatment. Her post-operative follow-up by CT scans and tumor markers at 3, 6, and 12 months was normal.
Figure 2: Right ovarian solid mass 11X9 CM with fallopian tube (Case 1).
Figure 3:Hematoxylene and Eosin picture (H&EX400) shows sheets of large cells with vesicular nuclei and mitotic activity separated by fibrous strands with lymphocytes (Case 1).
Figure 4: Immunohistochemical staining with CD 117 revealed diffuse positive membranous and cytoplasmic reaction (X 400) (Case 1).
Case II
A lady aged 25 years old, presented by primary infertility 3 years because of chronic anovulation diagnosed as PCOS based on chronic anovulation, ultrasound picture, and hyperandrogenism. Other fertility factors were normal. History of ovarian stimulation with clomiphene citrate and gonadotrphins for 8 months with poor response. The case was referred to our center for laparoscopic ovarian drilling. During routine ultrasound examination; left ovary showed a heterogenous ovarian mass 8X6 cm with a solid area more than 1 cm but no ascites and free pelvic and abdominal structures. Serum CA125 level was in normal range (12 IU/ml). After counseling of the couple; laparoscopy was performed (on 26/09/2006) during which the left ovarian mass could not be removed for fear of rupture of the capsule and spillage to pelvic cavity.
The case was shifted immediately to laparotomy for surgical staging. The tumor was limited to left ovary with intact capsule and free pelvic and abdominal organs. Intra-operative frozen section was done, and ovarian dysgerminoma was suspected. Left salpingo-oophorectomy, biopsies from other ovary, omental biopsy, peritoneal washing were done. The histopathological examination revealed left ovarian dysgerminoma, free other ovary, other biopsies and fluid cytology were free. The case was diagnosed as stage IA ovarian dysgerminoma and subsequently no further adjuvant treatment was given. During follow up with CT scans and tumors markers; the patient was going well with no evidence of tumor recurrence. One year later; the patient lost to follow up and her reproductive outcome was not known.
Discussion
Since introduction of in vitro fertilization in 1981 by Steptoe and Edward thousands of infertile couples could have children but ovaries of these women were subjected to ovarian stimulation over variable periods of time. There is a debate if the ovarian stimulating drugs increase risk of ovarian cancer or not. Some studies supported its role while others did not [3-7]. Moreover, progesterone, that usually used after ovarian stimulation as “luteal support” was reported to increase the risk for serous borderline ovarian tumors (RR 1.82; 95% CI 1.03-3.24), and among women treated with ≥4 cycles of progesterone (RR 2.63; 95% CI 1.04-6.64) [11]. On the other hand, other associated factors with infertility as anovulation, endometriosis, and nulliparity may increase risk of cancer. Factors suppressing ovulation, including pregnancy and oral contraceptive use, were inversely associated with the risk of all histologic types [12].
Yin et al., studied 14,764 women had been diagnosed with PCOS; 182 developed a primary cancer 1 year or more after PCOS diagnosis [13]. The fully adjusted HR was 1.15; 95% CI, 1.00-1.33. The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers. However, the study did not report the histopathologic types of the diagnosed cases of ovarian cancer and did not clarify if these women had received ovulation induction drugs. Besides the biological factors in PCOS, ovarian stimulation usually results in polyfollicular development with possibility of more ovulation traumas which is the basic part of pathogenesis of ovarian carcinoma according to “incessant ovulation theory” [1]. However, this is applied more for epithelial ovarian tumors.
In the first case, although rare, malignant ovarian tumors with pregnancy represent a real challenge. The reported incidence is 0.42 per 1000 pregnancies mainly borderline tumors followed by epithelial tumors [14]. However, diagnosis of ovarian mass was late at 22 weeks of pregnancy as the mass may mimic luteoma of pregnancy and corpus luteum hematoma .The diagnosis was supported by elevated LDH which agreed with other authors [15]. To the best of our knowledge, no published studies to report the association between ovarian stimulating drugs and malignant germ-cell tumors in women with PCOS. In case 2, ovarian tumor was diagnosed during routine transvaginal ultrasound before laparoscopic ovarian drilling for treatment of PCOS. The updated Cochrane review concluded increased incidence of ovarian cancer after fertility drugs but did not report specific increase in incidence of dysgerminoma [7]. Both cases were diagnosed at stage 1 (Ic and Ia respectively), Thomas and colleagues reported that approximately 65% of patients with ovarian dysgerminoma present with stage IA disease with a cure rate approaching 100%, and fertility is usually preserved [16].
Conclusion
We reported 2 cases of ovarian dysgerminoma in patients with PCOS following ovarian stimulation. A large multicenter observational study is recommended to investigate a possible association.
Abbreviations
IVF: In-vitro Fertilization
ICSI: Intracytoplasmic Sperm Injection
PCOS: Polycystic Ovarian Syndrome
GCT: Germ-Cell Tumor
LDH: Lactate Dehydrogenase
Acknowledgment
Not applicable.
Ethics Approval and Consent to Participate
The case presentations are anonymous, and no IRB approval is needed for publication of case reports in our institution. Figures are anonymous and do not point to patient personal information.
Consent
A written consent for publication was obtained from “case 1” and a verbal consent from “case 2” as she is travelling abroad nowadays.
Availability of Data and Material
The data and figures are included in the manuscript.
Competing Interests
None.
Funding
None.
Author Contributions
RH and NA: Collection of clinical data, management of the cases, and editing the manuscript.
AA and KZ: Histopathological diagnosis and preparation of histopathology pictures.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Fri 08, May 2020Accepted: Thu 21, May 2020
Published: Thu 28, May 2020
Copyright
© 2023 Reda Hemida. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.05.12
Author Info
Ahmed Said Khaled Zalata Naser El-lakkany Reda Hemida
Corresponding Author
Reda HemidaDepartment of Obstetrics and Gynecology, Mansoura University, Egypt
Figures & Tables

References
- Tung KH, Wilkens LR, Wu AH, McDuffie K, Nomura AM et al. (2005) Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am J Epidemiol 161: 321-329. [Crossref]
- Mandai M, Konishi I, Kuroda H, Fujii S (2007) LH/hCG action and development of ovarian cancer--a short review on biological and clinical/epidemiological aspects. Mol Cell Endocrinol 269: 61-64. [Crossref]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG (1994) Ovarian tumors in a cohort of infertile women. N Engl J Med 331: 771-776. [Crossref]
- Whittemore AS, Harris R, Itnyre J (1992) Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 136: 1184-1203. [Crossref]
- van Leeuwen FE, Klip H, Mooij TM, van de Swaluw AM, Lambalk CB et al. (2011) Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod 26: 3456-3465. [Crossref]
- Rizzuto I, Behrens RF, Smith LA (2013) Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev 13: CD008215. [Crossref]
- Rizzuto I, Behrens RF, Smith LA (2019) Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev 6: CD008215. [Crossref]
- Trikudanathan S (2015) Polycystic ovarian syndrome. Med Clin North Am 99: 221-235. [Crossref]
- Gottschau M, Kjaer S, Jensen A, Munk C, Mellenmkjaer L (2015) Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol 136: 99-103. [Crossref]
- Bjørnholt SM, Kjaer SK, Nielsen TS, Jensen A (2015) Risk for borderline ovarian tumours after exposure to fertility drugs: results of a population-based cohort study. Hum Reprod 30: 222-231. [Crossref]
- Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR et al. (2003) Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol 158: 629-638. [Crossref]
- Harris HR, Titus LJ, Cramer DW, Terry KL (2017) Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer 140: 285-291. [Crossref]
- Yin W, Falconer H, Yin L, Xu L, Ye W (2019) Association Between Polycystic Ovary Syndrome and Cancer Risk. JAMA Oncol 5: 106-107. [Crossref]
- Machado F, Vegas C, Leon J, Perez A, Sanchez R et al. (2007) Ovarian cancer during pregnancy: analysis of 15 cases. Gynecol Oncol 105: 446-450. [Crossref]
- Yoshimura T , Takemori K, Okazaki T, Suzuki A (1988) Serum lactic dehydrogenase and its isoenzymes in patients with ovarian dysgerminoma. Int J Gynaecol Obstet 27: 459-465. [Crossref]
- Thomas GM, Dembo AJ, Hacker NF, De Petrillo AD (1987) Current Therapy for Dysgerminoma of the Ovary. Obstet Gynecol 70: 268-275. [Crossref]
