Malignant Melanoma of Adrenal Gland: A Case Report
A B S T R A C T
Primary adrenal melanoma (PAM) is a rare malignant tumor, with few reported cases in the published literature and often correlated with high mortality rates. This condition usually has an unclear clinical presentation and must have its differential diagnosis regarding metastatic processes from malignant melanoma and pigmented pheochromocytoma. In this article, we report a case of a 49-year-old male diagnosed with PAM, alongside with its progress and discussions about the diagnostic criteria established, willing to expand and disseminate knowledge and awareness about PAM [1].
Keywords
Adrenal gland, melanoma, primary
Introduction
Although malignant melanomas themselves are not rare conditions, primary adrenal malignant melanomas are an exceptionally rare mortal pathological entity that, despite being rare and mortal, has just a few reports in the literature. Its etiology remains unknown; however, it is suspected that high Adrenocorticotropic Hormone (ACTH) concentrations are a predisposing factor that leads to the development of this condition. The characteristics of clinical signs and symptoms are not clear and evident; however, the most common symptom is flank pain, being impossible to obtain typical features from imaging examination [2-6].
To establish the diagnosis, these criteria must be met: a single adrenal should be affected; other possible sources of melanocytic tumors must be excluded; no evidence of endocrine disturbances must be present; the histologic sample should be characteristic; no immunohistochemical endocrine markers should be positive; no neurosecretory granules must be identified in the electron microscopy technique. Nonetheless, Carstens et al. criteria´s were defined by only one adrenal gland involved; a careful examination to eliminate melanoma in other parts of the patient’s body; no surgery history of pigmented mucosa, cutaneous, or ocular lesions, ruling out any hidden pigmented lesions, preferably by autopsy results [6-13]. From 1946 to 2017, only 14 cases of patients suffering from Primary adrenal melanoma were retrieved in MEDLINE.
Case Report
A 49-year-old male with no relevant background history. His current condition started 3 months prior to his consultation, in which he referred generalized colicky abdominal pain, weight loss of 4 kg in 4 months, asthenia, and adynamia. Computed Axial Tomography was performed, reporting a 12.2×11×8.1 cm retroperitoneal tumor with multiple pre-aortic lymph nodes. It was located posterior to the pancreas, left lateral to the aorta, and superior to the left renal vessels, infiltrating the gastric fundus but not affecting the spleen.
On 06/09/2016, the patient underwent an exploratory laparotomy, and an intraoperative biopsy was performed. The results revealed positive malignant rhabdoid tumor findings, ruling out melanoma. The voluminous and retroperitoneal tumor infiltrated the gastric fundus and cardiac regions, along with the superior pole of the spleen, diaphragm, and the pancreatic tail. As a conduct, en bloc resection was performed with total gastrectomy, left adrenalectomy, splenectomy, partial resection of the left diaphragm, and distal pancreatectomy. Furthermore, both esophagojejunal and jejuno-jejunal anastomoses without complications were performed.
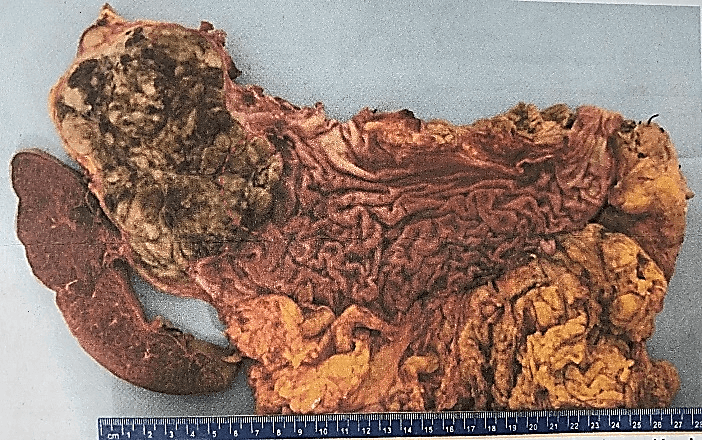
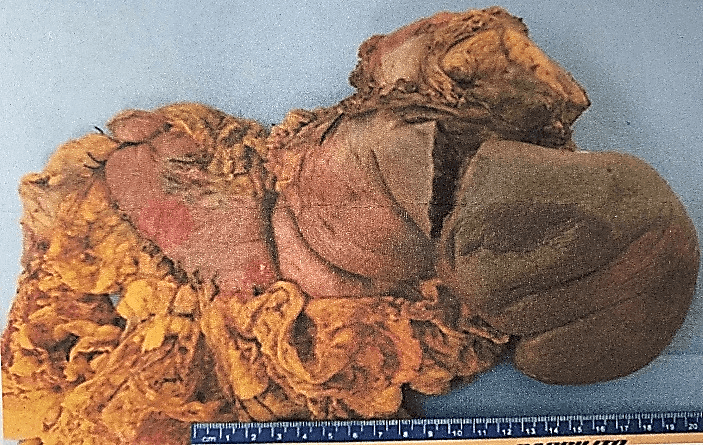
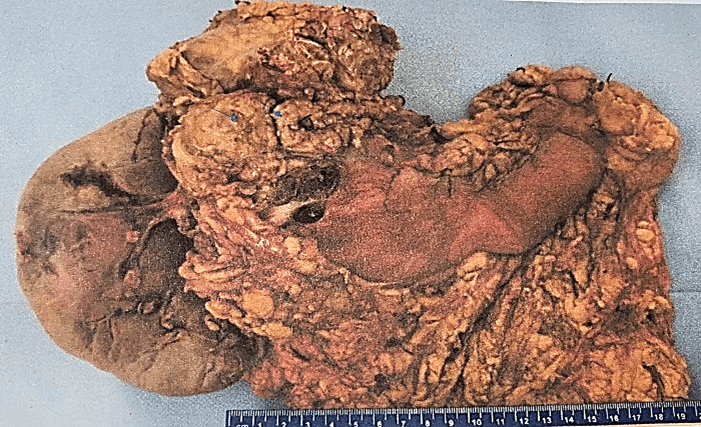
The definitive histopathological report revealed a malignant epithelioid melanoma originated from the medulla, measuring 12×10.5cm and associated with a high mitotic rate (10 mitoses/mm²). The report also confirmed infiltration and perforation of the adrenal capsule extending to the retroperitoneal soft tissues, as well as infiltration and ulceration of the gastric wall, perineural and lymphovascular invasion. Additionally, the diaphragmatic border was positive for neoplasia, the pancreatic was negative at 2 cm, the proximal gastric was negative at 3.5 cm, and the distal gastric was negative at 17 cm. The marginal or peripheral border was negative at 1 mm and 30 lymph nodes were negative for metastasis (Figures 1-3). Both pancreas and spleen didn’t have any neoplastic lesions. Lastly, the immunohistochemical analysis reported positive immunostaining for HMB-45 and S-100, but negative for synaptophysin. Therefore, the diagnosis followed the recommended criteria.
Figure 1: Aspect of the specimen to the section, the red circle highlights the area of ulceration of the gastric wall.
Figure 2: Aspect of the specimen from its anterior face.
Figure 3: Aspect of the specimen from its posterior face, the red arrows indicate the diaphragmatic border, the blue arrows highlight the pancreatic border.
Six weeks after the surgery was performed, the patient was treated with adjuvant treatment using annual Interferon alfa-2b annually 20M IU/m². At seven weeks after the surgery, he was assessed by the radiation oncology department, deciding on 3D hypofractionated radiotherapy treatment at the tumoral bed, with doses of 3750 CGY in 15 fractions. Moreover, nine weeks after the surgery, the patient was admitted due to seizures and suspected progression to the central nervous system (CNS), which was confirmed by extension studies, revealing tumor activity only in the CNS. Hence, holocranial radiotherapy was administered at doses of 2000 CGY in 5 fractions, to which the patient responded adequately without presenting symptoms. Then, due to progression, the medical oncology decided to treat with temozolomide 200 mg/m² D1-D5 every 28 days. However, eight months after the beginning of radiotherapy treatment, the patient died. No local recurrence was documented.
Discussion
The presence of a large inhomogeneously enhancing mass with multiple focal low-density areas arising in the location of the adrenal bed suggests a malignant process, such as adrenal cortical carcinoma or metastases, which are originated from a primary tumor such as bronchogenic carcinoma, melanoma or possibly lymphoma [4].
Primary adrenal melanoma is usually a larger tumor with the largest diameter ranging from 8 to 17 cm, being mostly a nonfunctional tumor that only invades the unilateral gland. Middle-aged and old people are more susceptible to developing this condition. Related studies suggest that it might occur due to pluripotent neural crest cells, which could migrate after induction and enter into the multicell lineage, including melanocytes, neurons, glial cells of the peripheral nervous system, and adrenal chromaffin cells through cell differentiation. Melanocytes could be located in the adrenal gland and cause metaplasia and malignant transformation, leading to the formation of the melanoma [9].
The histological differentiation between primary adrenal melanoma and pigmented pheochromocytoma is very difficult, some authors have frequently observed the presence of melanin or a melanin-like pigment in pheochromocytoma. Dao et al. consider that adrenal melanoma arises from pheochromocytoma and should be called melanotic malignant pheochromocytoma; otherwise, Zalatnai et al. consider that pheochromocytoma and adrenal melanoma are two different entities. The differential diagnosis between primary adrenal melanoma and pigmented pheochromocytoma rests on immunohistochemical staining such as: neuroendocrine markers like synaptophysin, chromogranin and neuron-specific enolase and on electronic microscopy techniques to see neurosecretory granules. It has been observed that neuroendocrine markers and neurosecretory granules are only present in pheochromocytoma [12].
Primary lesions could be found in patients with adrenal metastases of malignant melanoma. The characteristic of the Computed Tomography (TC) scanning of adrenal metastases display largely adrenal heterogeneous lesions with central or irregular areas of necrosis/hemorrhage and a thick contrast-enhancing rim. Bilateral metastasis with characteristic appearance is a kind of adrenal metastasis. It may be also misdiagnosed as PAM because of the hidden melanoma of the skin or other parts, and surgical history of pigmented mucosa, skin, or ocular lesions. Pigmented pheochromocytoma, pigmented adrenal adenoma, adrenal hematoma, and adrenocortical carcinoma also must be considered in the differential diagnosis which mainly depends on immunohistochemistry and electron microscopy techniques [11].
Conclusion
As stated in this study, primary adrenal melanoma is a rare and mortal condition, which has few reports and studies about it in the literature and due to its clinical presentation, can be mistaken for metastatic processes and pigmented pheochromocytoma. In this way, the diagnosis of PAM needs to follow specific criteria and it is almost always done by exclusion.
This case report is coherent with the others published in the literature, as the patient was diagnosed by following the established criteria and the treatment followed the guidelines. Since PAM is a condition with high lethality rates, despite the effort, the patient succumbed to the disease, which is the average expected outcome. Lastly, although published studies have already shed more light on the importance of studying PAM, more studies and research are needed. It is particularly important to understand this condition, so it is possible to develop more specific diagnostic methods and unravel many other questions and doubts regarding primary adrenal melanoma.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Sat 19, Mar 2022Accepted: Mon 04, Apr 2022
Published: Wed 20, Apr 2022
Copyright
© 2023 Frigerio Pamela. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2022.01.01
Author Info
Alvarado Alvarez Hermes Marco Tulio Frigerio Pamela Maria Eduarda Silva Dias Alvarez Castro Jose Alfonso
Corresponding Author
Frigerio PamelaSurgical Residente, Hospital Universitario de Saltillo Universidad Autónoma de Coahuila, Mexico
Figures & Tables
References
1. Zalatnai A, Szende
B, Tóth M, Rácz K (2003) Primary malignant melanoma of adrenal gland in a
41-yr-old woman. Endocr Pathol 14: 101-105. [Crossref]
2. Granero LE, Al Lawati
T, Bobin JY (2004) Primary melanoma of the adrenal gland, a continuous dilemma:
Report of a case. Surg Today 34: 554-556. [Crossref]
3. Liatsikos EN,
Papathanassiou Z, Voudoukis T, Repanti M, Sklavou C et al. (2006) Laparoscopic adrenalectomy
in a patient with primary adrenal malignant melanoma. J Endourol 20:
123-126. [Crossref]
4. Bastide C, Arroua F, Carcenac A, Anfossi E, Ragni E et al. (2006) Primary
malignant melanoma of the adrenal gland. Int J Urol 13: 608-610. [Crossref]
5. Villers A, Lami MC,
Filoche P, Milinkevitch S, Goujon JM et al. (2006) [Regression of primary
melanoma with metastases associated with amyopathic dermatomyositis]. Ann
Dermatol Venereol 133: 573-576. [Crossref]
6. Uras A, Budak D,
Ariogul O, Görpe A, Tahsinoǧlu M (1978) A functioning black adenoma of the
adrenal gland. Clin Oncol 4: 181-186. [Crossref]
7. González Sáez L,
Pita Fernández S, Lorenzo Patĩo MJ, Arnal Monreal F, MacHuca Santacruz J et al.
(2011) Primary melanoma of the adrenal gland: A case report and review of the
literature. J Med Case Rep 5: 273. [Crossref]
8. Sasidharan K, Babu
AS, Pandey AP, Rao MM, Walter A (1977) Primary melanoma of the adrenal gland: A
case report. J Urol 117: 663-664. [Crossref]
9. Xu B, Hong Y, Jin M, Li M, Wang C et al. (2017) Primary adrenal malignant
melanoma: A case report and review of literature. Medicine (Baltimore)
96: e8956. [Crossref]
10. Parker LA, Vicent
LM (1986) Detection of adrenal melanoma with computed tomography. Urol
Radiol 8: 209-210. [Crossref]
11. Amérigo J, Roig J,
Pulido F, Belda R, Vázquez Ramírez FJ et al. (2000) Primary malignant melanoma
of the adrenal gland. Surgery 127: 107-111. [Crossref]
12. Dick JC, Ritchie GM, Thompson H (1955) Histological differentiation between phaeochromyocytoma and melanoma of the suprarenal gland. J Clin Pathol 8: 89-98. [Crossref]
13. Carstens PH, Kuhns JG, Ghazi C (1984) Primary malignant melanomas of the lung and adrenal. Hum Pathol 15: 910-914. [Crossref]
