Live Cesarean Scar Pregnancy: A Case Report
A B S T R A C T
Introduction: The overall incidence of Cesarean scar pregnancy is increasing due to Cesarean rates. This life-threatening condition has been historically managed in various ways as no single modality is reliable enough. We report this case of live CSP managed initially with Fetocide followed by Methotrexate but requiring Surgical management later on.
Presentation: A 32 years old para 5 with four previous Cesarean sections was diagnosed with live CSP. HCG level was 76,619. The initial management was fetocide with KCL followed by Methotrexate. The treatment was considered successful in view of appropriate reduction in serum HCG levels. The woman required surgical management 10 weeks after the initial management, but the blood loss was minimal.
Discussion: A CSP may be asymptomatic or present with non-specific symptoms. The rate of initial misdiagnosis is as high as 76%. TVUSS enables correct CSP diagnosis and implementation of minimally invasive effective treatment. HCG levels can affect the overall outcome, but medical management can be considered even with high HCG levels.
Conclusion: CSP is a life-threatening condition, therefore timely diagnosis and appropriate management is crucial. Medical management can be considered in most cases even with high HCG, but management has to be tailored according to the patient. Close follow up of patient after Medical treatment is important as they may require further intervention.
Keywords
Ectopic, cesarean scar pregnancy, early pregnancy, bleeding, ERPC
Introduction
A pregnancy that occurs outside of the uterine cavity is known as ectopic pregnancy [1]. The overall prevalence of ectopic pregnancy is approximately 2%. In most cases (about 97%), an ectopic pregnancy is located in a fallopian tube [2]. However, an ectopic pregnancy can occur in other anatomic locations including the myometrium, cervix, ovaries, and abdomen. Caesarean scar pregnancy (CSP) is a type of ectopic pregnancy where the fertilized egg is implanted in the muscle or fibrous tissue of the scar after a previous caesarean section [2]. This is a life-threatening form of pregnancy due to the increased risk of uterine rupture and haemorrhage that may require an urgent hysterectomy and blood transfusion [3]. The frequency of caesarean scar pregnancy is reported to be 1:1,800 to 1:2,226 (0.05-0.04%) of all pregnancies. In women after a caesarean section, the frequency of CSP is approximately 0.15%, which constitutes 6.1% of all ectopic pregnancies in patients after at least one caesarean operation [4].
Previous dilation and curettage, caesarean sections, trauma and myomectomies are risk factors that may contribute to the formation of a caesarean scar ectopic [5]. Despite more than half of these patients experiencing greater than 2 caesarean deliveries, the risk for a caesarean scar ectopic does not necessarily increase with the number of caesarean deliveries [6]. A recent systematic review found that 52% cases followed one previous caesarean section, 36% after two and 12% after three or more previous caesarean sections [7].
Risk for caesarean section scar implantation has not been correlated to single versus double layer closure of the hysterotomy at the time of caesarean section. Caesarean scar implantation may be more common following caesarean sections for elective indications, which is theorized to be due to impaired healing of an unlaboured lower uterine segment [8]. The case mentioned below shows how early diagnosis and timely management can prove beneficial in appropriately managing this condition.
Case Report
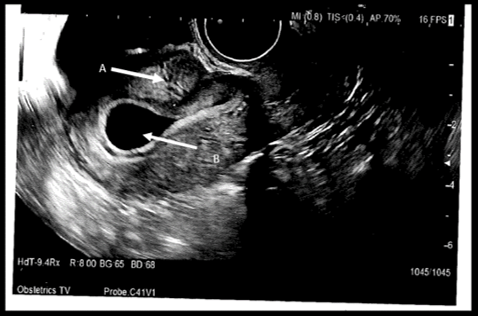
A 32-year-old Gravida 7 para 5 presented with PV spotting in early pregnancy. She had a background of Graves’ disease, Asthma, Transverse myelitis, mild gastritis, depression, Irritable bowel syndrome and chronic intractable pain. Her regular medications included Sertraline 50mg OD, Duloxetine 30mg OD, Domperidone 10mg TDS (for nausea), Fostair 100mcg/dose, Metoclopramide 10mg TDS, Folic acid 5mg OD, Ferrous fumarate, Beconase aqueos 50mcg/dose nasal spray and Buscopan 10mg TDS. Her obstetric history included 1 early medical TOP (Termination of pregnancy), 1 NVD with a 4th degree tear followed by four Elective- C-sections. Her age of menarche was 15years, menstrual cycle was regular, and she was up to date with her cervical smears. She attended pregnancy advisory clinic for a TOP around 6 weeks of gestational amenorrhoea. As per protocol, she had USS to confirm it was intra-uterine. Her first Transvaginal USS done at 6 weeks of pregnancy showed: absent fetal pole, 5.7mm * tear drop shaped Gestational sac (GS) within the uterine cavity but not at fundus. The GS extended to near C-section scar (Figure 1).
Figure 1: TransVaginal USS Image of Caesarean scar pregnancy (CSP). A) Arrow pointing towards CSP. B) Arrow pointing towards Empty uterine cavity at fundus.
A repeat Trans vaginal USS at eight weeks confirmed live C-section scar ectopic. The diagnosis was explained to the patient along with possible management options which included:
1. KCL injection in the GS, followed by consideration of systemic Methotrexate +/- Mifepristone, if Beta HCG levels drop.
2. USS + Hysteroscopic guided evacuation along with Interventional radiology+/- Uterine artery embolization (UAE), if there are any concerns regarding bleeding or the results with MTX are not satisfactory. Alternatively, only UAE could be considered.
A joint decision in favour of the first management plan took place between the Health care professionals and the patient. Fetocide was performed with TV USS guided injection of 1.5mls of 15% KCL through the left fornix 2cm from vagina to fetus. The patient experienced immediate post OP tugging pain in C-section scar after fetocide. Repeat USS the next day confirmed Intrauterine death. The patient was given 110mg Methotrexate intramuscularly on day 1 of fetocide as B-hcg dropped. She was discharged the next day but presented 3 times in a space of two weeks struggling with abdominal pain. During these visits her Haemoglobin was stable and there was no PV bleeding or signs of significant intra-abdominal bleeding, therefore she was managed conservatively with analgesia. Her Beta HCG plummeted from initial level of 76,619 to 69601 on day 1, 68556 on day 4 to 42413 on day 7. She represented around 10 weeks post MTX with heavy PV bleeding and had emergency evacuation of Retained products under GA, but the estimated blood loss was only 200mls. Her Beta-HCG dropped down to 25 in 8.5 weeks This case is a good example of timely diagnosis and appropriate management of a potentially life-threatening condition with minimal blood loss.
We had two other cases of CSP with Beta HCGs of 17,036 and 974 at diagnosis both successfully managed with Methotrexate, not requiring subsequent intervention/emergency admissions. Another case of CSP with Beta HCG of 13884 at diagnosis was managed by Evacuation of retained products without Methotrexate, but the woman lost 1.5 Litres of blood during the operation and was controlled with the use of uterotonics, Tranexemic acid and mechanical pressure on bleeding site with Foley’s balloon catheter.
Discussion
In this case, we had a young woman with four previous caesarean deliveries who presented for TOP and could have been subjected to significant harm without TVUSS, hence the importance of USS before offering TOP. A diagnosis of caesarean scar pregnancy based on symptoms and pelvic examination alone is difficult as CSP is asymptomatic in its initial phases. Later, signs of this type of pregnancy like vaginal bleeding and abdominal pain are frequently non-specific and also often present in other obstetric conditions [2]. Early CSP is frequently misdiagnosed as normal intrauterine pregnancy, missed abortion, inevitable abortion, gestational trophoblastic disease or cervical pregnancy [9]. As pregnancy develops, the diagnosis of CSP on US becomes more difficult. In early pregnancy, US enables correct CSP diagnosis and implementation of minimally invasive effective treatment. However, in advanced pregnancy (above week 12), US (usually transabdominal) produces images that are difficult to interpret, and final diagnosis is possible only during surgery.
However, because of difficult diagnoses, the misdiagnosis rate of CSP at first consultation is as high as 76% [10]. It can cause uterine or unmanageable bleeding if it is not treated in a time or in an improper way like curettage, sometimes making hysterectomy a must to control the life threatening haemorrhage resulting in loss of reproductive function [6, 10]. The scar ectopic pregnancy in our case was diagnosed with the help of transvaginal USS only, thus avoiding unnecessary further investigations.
Although CSP is a rare form of ectopic pregnancy, the incidence is no doubt increasing with rising Caesarean deliveries and therefore signifies the importance of high clinical suspicion [11]. Transvaginal Ultrasonography is necessary, and it is the simplest and most practical method with an accuracy of 84.6% in diagnosing CSP. Suggested diagnostic criteria for CSP include confirmation by transvaginal ultrasound based on the following reasons: A gestational sac is located anteriorly at the uterine isthmus within a visible myometrial defect at the site of a previous lower-segment Caesarean section delivery scar; An empty uterine cavity and cervical canal are found; Evidence of a functional trophoblastic/placental circulation on colour Doppler examination [12].
A new USS grading system for CSP was proposed recently by shin-Yu Lin et al, 2018 [13]. This categorised them into four grades depending upon the ultrasound description of the extent of entrenchment of GS through the Myometrium (Figure 2). However, whether it can be used internationally as a standard classification is not clear yet. This is a good effort at proposing a possible management pathway to a life-threatening condition.
Figure 2: Grade I CSP represented the depth of CSP embedded in less than one-half thickness of the lower anterior corpus. Grade II CSP implied CSP occupied more than one-half thickness of the lower anterior corpus. In grade III CSP, the GS bulged out the overlying myometrium and uterine serosa. In grade IV CSP, the GS became an amorphous tumour with rich vascularity at the caesarean scar.
According to this study Logistic regression analysis illustrated that a higher clinical grade on USS description was associated with greater invasiveness of the surgical procedure. However, the same study suggested that neither this grading system nor the demographic parameters could predict successful treatment with Systemic Methotrexate. Although transvaginal USS remains the primary imaging modality for this diagnosis, MRI may be useful in the setting of equivocal cases and may help in the detection of placental implantation or bladder invasion [14].
Level of β-HCG has an important reference value before and after the treatment of CSP. Several studies suggested better efficacy with Medical treatment in those with a Beta HCG of <5000miu/ml [15, 16]. However, it can be considered in patients with higher levels of Beta HCG as well. As in the case mentioned above the initial Beta HCG was 76616. Conservative treatment is appropriate for women who are pain free and hemodynamically stable with an unruptured CSP of <8 weeks’ Gestation and a myometrial thickness <2mm between CSP and the bladder as there is high probability that trophoblast reaches the vesico-uterine space on the bladder wall, in thin myometrium and predispose to more surgical complications [7].
On the other hand, rupture of the scar and heavy bleeding may occur following medical treatment, as well, as described by Jurkovic et al..[17]. Based on this fact some authors had proposed that the medical approach should be combined with either bilateral uterine artery embolization or vasopressin intracervical injection combined with 18 French Foley catheter balloon tamponade, thus avoiding such complications [18, 19]. Surgical treatment options include hysteroscopy for visualization of the uterine cavity combined with incision and aspiration of the ectopic mass by operative laparoscopy. Aspiration of a very small gestational sac may facilitate pregnancy absorption [17, 20].
Trans cervical complete aspiration of the gestational sac under ultrasound guidance can also be performed without any complementary medical treatment [7]. Dilatation and curettage should not be considered as the first choice of therapy [17, 21]. This is because the majority of the villi are implanted in the myometrium and it seems very unlikely that the gestational sac could be expelled by curettage without perforating the uterine wall or damage to the urinary bladder, an accident that may cause life threatening bleeding and require emergency laparotomy [11, 22]. The surgical approach is supported by others even if the patient is not bleeding [11, 18]. This includes elective laparotomy and wedge excision of the gestational mass when fertility is to be conserved. Several of these authors believe that even if recurrence is unlikely, the resection of the old scar with a new uterine closure can minimize the risk of recurrence [11]. Wedge resection may, however, result in postoperative adhesions and fertility may be affected. In order to preserve fertility and reduce morbidity, surgery has been combined with selective embolization of the uterine arteries [21].
Conclusion
This case was a good example of timely diagnosis and appropriate treatment of CSP. It, however, highlights the importance of close follow up as the woman required (ERPC) ten weeks after Methotrexate. Although this woman required subsequent surgical management, initial Methotrexate helped to reduce the overall blood loss as compared to the other cases where surgical management was considered initially. Considering the very high initial Beta HCG, the overall outcome of the case was good. However, no single modality (Medical/Surgical/conservative) is entirely reliable and none can guarantee uterine integrity. Treatment policy should be tailored to each patient and the viability of the pregnancy, gestational age as well as future family planning need to be taken in consideration.
Article Info
Article Type
Case ReportPublication history
Received: Sat 04, Jan 2020Accepted: Wed 22, Jan 2020
Published: Fri 31, Jan 2020
Copyright
© 2023 Zohra Amin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CROGR.2019.01.04
Author Info
Corresponding Author
Zohra AminTrainee Obs/Gynecology, Derriford Hospital, Plymouth, UK
Figures & Tables

References
- Barnhart KT, Franasiak JM (2018) Tubal Ectopic Pregnancy. Obstet Gynecol 131: E91-E103.
- Pędraszewski P, Wlaźlak E, Panek W, Surkont G (2018) Cesarean scar pregnancy - a new challenge for obstetricians. J Ultrason 18: 56-62. [Crossref]
- Hashimi S, Maiti S, Macfoy D (2012) Successful conservative management of ectopic pregnancy in caesarean section scar. BMJ Case Rep 2012: bcr2012006925. [Crossref]
- Ash A, Smith A, Maxwell D (2007) Caesarean scar pregnancy. BJOG 114: 253-263. [Crossref]
- Fait G, Goyert G, Sundareson A, Pickens A Jr (1987) Intramural pregnancy with fetal survival: case history and discussion of etiologic factors. Obstet Gynecol 70: 472-474. [Crossref]
- Rotas MA, Haberman S, Levgur M (2006) Cesarean scar ectopic pregnancies etiology. Obstet Gynecol 107: 1373-1381. [Crossref]
- Maymon R, Halperin R, Mendlovic SE, Schneider D, Herman A (2004) Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Hum Reprod Update 10: 515-523. [Crossref]
- Panelli DM, Phillips CH, Brady PC (2015) Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract 1: 15. [Crossref]
- Zhang Y, Gu Y, Wang JM, Li Y (2013) Analysis of cases with caesarean scar pregnancy. J Obstet Gynaecol Res 39: 195-202. [Crossref]
- Baradwan S, Khan F, Al-Jaroudi D (2018) Successful management of a spontaneous viable monochorionic diamniotic twin pregnancy on caesarean scar with systemic methotrexate: A case report. Medicine (Baltimore) 97: e12343. [Crossref]
- Jurkovic D, Knez J, Appiah A, Farahani L, Mavrelos D et al. (2016) Surgical treatment of Caesarean scar ectopic pregnancy: efficacy and safety of ultrasound-guided suction curettage. Ultrasound Obstet Gynecol 47: 511-517. [Crossref]
- Fylstra DL (2012) Ectopic pregnancy not within the (distal) fallopian tube: etiology, diagnosis, and treatment. Am J Obstet Gynecol 206: 289-299. [Crossref]
- Li-Ping Fu (2018) Therapeutic approach for the caesarean scar pregnancy. Medicine (Baltimore) 97: e0476. [Crossref]
- Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN et al. (2018) New ultrasound grading system for caesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS One 13: e0202020. [Crossref]
- Brancazio S, Saramago I, Goodnight W, McGinty K (2019) Caesarean scar ectopic pregnancy: Case report☆. Radiol Case Rep 14: 354-359. [Crossref]
- Shufaro Y, Nadjari M (2001) Implantation of a gestational sac in a caesarean section scar. Fertil Steril 75: 1217. [Crossref]
- Ravhon A, Ben‐Chetrit A, Rabinowitz R, Neuman M, Seller U (1997) Successful methotrexate treatment of a viable pregnancy within a thin uterine scar. Br J Obst Gynaecol 104: 628-629. [Crossref]
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R et al. (2003) First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 21: 220-227. [Crossref]
- Chuang J, Seow KM, Cheng WC, Tsai YL and Hwang JL (2003) Conservative treatment of ectopic pregnancy in a caesarean section scar. Br J Obstet Gynecol 110: 869-887.
- Ghezzi F, Lagana D, Franchi M, Fugazzola C and Bolis P (2002) Conservative treatment by chemotherapy and uterine arteries embolization of a cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol 103: 88-91. [Crossref]
- Zhang G, Li J, Tang J, Zhang L, Wang D et al. (2019) Role of collateral embolization in addition to uterine artery embolization followed by hysteroscopic curettage for the management of cesarean scar pregnancy. BMC Pregnancy Childbirth 19: 502. [Crossref]
- Shih JC (2004) Cesarean scar pregnancy: diagnosis with three-dimensional (3D) ultrasound and 3D power Doppler. Ultrasound Obstet Gynecol 23: 306-307. [Crossref]
