Letrozole-Induced Acute Interstitial Nephritis
A B S T R A C T
Background: Acute interstitial nephritis is often associated with drug therapy. It can be a common cause of acute kidney injury, traditional chemotherapy agents have been associated with various forms of renal damage, but this is not commonly seen with anti-hormone therapy. Here we report a case of aromatase inhibitor associated acute interstitial nephritis with the use of letrozole.
Case Presentation: We present the case of an 84-year-old female commended on letrozole for the management of estrogen sensitive breast cancer. The patient developed oliguric acute kidney injury 3-weeks after initiation, with a creatinine rise to 1069µmol/L, from a previously normal baseline. She had no response to hydration and underwent a renal biopsy which demonstrated acute interstitial nephritis. She was commended on 4 weeks of oral prednisone starting at 20mg with a tapering dose. Renal function returned to baseline on discharge and her aromatase inhibitor was ceased.
Conclusions: This is the first reported case of letrozole induced acute interstitial nephritis. It appears the disease was steroid responsive however it is unclear if this is a class effect of aromatase inhibitors or letrozole alone.
Keywords
Acute interstitial nephritis, letrozole, aromatase inhibitor
Background
Acute interstitial nephritis (AIN) is a common cause of acute kidney injury. It is defined as an immune-mediated response in the tubulointerstitium of the kidney and is usually associated with medications or infection [1, 2]. It is often difficult to distinguish clinically from other forms of acute kidney injury and the classic triad of rash, sterile pyuria and eosinophilia is frequently absent [1]. A history of new medication changes or recent illness increases suspicion for AIN, however renal biopsy remains the gold standard for diagnosis. The use of non-steroidal anti-inflammatory drugs, proton pump inhibitors and antibiotics are commonly associated with AIN [2]. Letrozole is a nonsteroidal competitive inhibitor of the aromatase enzyme system widely used in the treatment of hormone sensitive breast cancer in postmenopausal women. Although letrozole has not previously been associated with interstitial nephritis, the related aromatase inhibitor (AI) anastrazole has been reported to associate with crescentic glomerulonephritis [3]. Here we present the first case of letrozole associated AIN which appeared to be steroid responsive.
Case Presentation
We present the case of 84-year-old women with a background of locally invasive oestrogen receptor positive, Herceptin-2 negative breast cancer treated with lumpectomy, radiotherapy and oral letrozole. She had history of hypertension, cerebral vascular disease and hyperlipidaemia. She was admitted under the care of the Canberra hospital nephrology unit after presenting with acute kidney injury (AKI). A medication history revealed she had been on long term combination telmisartan/hydrochlorothiazide and atorvastatin. However, denied the use of any non-steroidal anti-inflammatory drug use or proton pump inhibitor use.
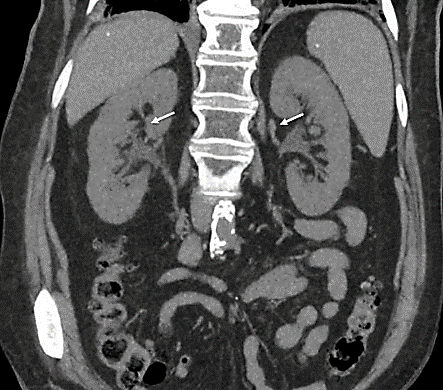
She had no previous underlying kidney disease, with a baseline creatinine level of 68µmol/L three weeks prior to presentation. On presentation the creatinine and urea were 1069µmol/L and 39.4 mmol/L (Table 1). She reported progressive oliguria and orthopnoea in addition to uremic symptoms of nausea and anorexia 3-weeks after commencing letrozole. Renal imaging demonstrated no evidence of urinary tract obstruction (Figure 1). Urinalysis demonstrated sterile pyuria, minimal proteinuria (0.8g/L) and no haematuria (Table 2). Autoimmune serology was negative, serum protein electrophoresis was normal and urine electrophoresis revealed 0.61g/L of protein consisting of mixed glomerulotubular proteins (Tables 2 & 3).
Table 1: Renal function over time for patient.
|
|
Baseline 19/04/2018 |
On presentation 13/05/2018 |
Post treatment 22/06/18 |
|
Serum Creatinine |
68 µmol/L |
1069 µmol/L |
88 µmol/L |
Table 2: Urine assessment on presentation.
|
ANCA |
Not Detected |
|
Anti-nuclear antibodies |
Not Detected |
|
ENA |
Negative |
|
dsDNA |
Negative |
|
Rheumatoid factor |
Negative |
|
Anti CCP antibodies |
Negative |
|
Free kappa to lambda light chains ratio |
Normal |
|
C3 |
Normal |
|
C4 |
Normal |
Table 3: Autoimmune screen at presentation.
|
Protein concentration |
0.85 (ref <0.14) |
|
Creatinine |
4.0 |
|
Protein to creatinine ratio |
212 |
|
Leucocytes |
>100 x 106/L |
|
Erythrocytes |
>100 x 106/L |
|
Squamous epithelial cells |
<10 x 106/L |
|
Nitrite |
Negative |
|
Casts |
Nil |
|
Red cell morphology |
Normal |
|
Electrophoresis |
Mixed glomerulotubular protein |
|
Immunofixation |
No monoclonal immunoglobulin protein detected |
The patient was initially treated with intravenous rehydration in the context of anorexia; however, the serum creatinine did not improve. Subsequently the patient went on to develop pulmonary oedema, which was only partially responsive to intravenous furosemide. Given her age and burden of malignant disease, the patient expressed she did not wish to proceed with dialysis however was agreeable to a renal biopsy pending her ability to lay prone. On the day of the biopsy, she received further diuresis to improve her fluid status. A biopsy was subsequently performed, without any complications. Results of the biopsy demonstrated an interstitial infiltrate of mononuclear cells including eosinophils consistent with acute tubulo-interstitial nephritis on light microscopy (Figure 2). Her Immunofluorescence was negative, and the electron microscopy showed no glomerular damage or deposition.
Figure 1: CT scan of the kidney, ureters and bladder showing no obstruction to be the cause of the patient acute kidney injury.
Figure 2: Renal biopsy: interstitial infiltrate of mononuclear cells including eosinophils consistent with acute tubulo-interstitial nephritis on light microscopy. No features of acute tubular necrosis or other glomerular damage. No other cause for acute renal injury identified.
The patient’s medication and disease history were reviewed, and the only new agent prescribed was the letrozole. The final diagnosis was consistent with a drug induced (DI) acute interstitial nephritis. After discussion with her medical oncologist the letrozole was ceased and the patient was commenced on 20mg daily of oral prednisolone along with histamine receptor blocker for gastrointestinal protection. The patient spent another 4 weeks in hospital undergoing rehabilitation due to deconditioning throughout her admission. Upon discharge her renal function retuned to base line, with a creatinine of 88 µmol/L (Table 1), her prednisolone was ceased, and she required nursing home placement due to social reasons. Her cancer was further managed with radiotherapy alone and the patient understood the risk of metastasizes.
Discussion and Conclusion
Drug induced AIN appears to be more prevalent with new cancer treatments [4]. There is now immunotherapy induced AIN noted amongst Programmed cell death protein 1 (PD-1) inhibitors, unlike the other traditional chemotherapeutic agents where we see acute tubular necrosis, glomerulonephritis or thrombotic microangiopathy [4, 5]. This case report highlights the risk of drug induced AIN with letrozole. It remains unclear if this is class effect of AIs or letrozole specific. It is thought the risk of AIN with antineoplastic agents is higher in patients with pre-existing chronic kidney diseases, however this was not the case with our patient [4]. This case we saw the patients renal function recover with the withdrawal of her letrozole but also the addition of corticosteroids. The use of corticosteroids in AIN remains unclear, most of the evidence is from retrospective studies [6]. However, the early initiation of corticosteroids in patients with drug induced AIN in the absence of significant fibrosis on renal biopsy seems a reasonable approach to reduce inflammation.
A case series by Prendecki et al., 2017, showed the initiation of corticosteroids in AIN had better outcomes on long term renal function in patients treated compared to those where only the offending agent was withdrawn. These results coincide with other smaller studies suggesting the use of steroids may have long term effect on renal function [6-8]. Duration of steroid therapy remains unclear; however, our patient was treated for a total of 4 weeks with a tapering dose of oral prednisolone. Evidence suggested therapy should not extend beyond 6 weeks, and either pulsed methylprednisolone with oral steroids or oral prednisolone alone are reasonable approaches [7]. Oral Steroid dosing is recommended to be weight based at 1mg/kg, we used a low dose of oral prednisolone and discontinued the letrozole [8]. Our patient had a marked response to treatment and preadmission medications were restarted during her admission including her combination telmisartan/hydrochlorothiazide with no effect on renal function.
This is the first known reported case of interstitial nephritis associated with aromatase inhibitors. We highlight the importance of considering interstitial nephritis due to letrozole or related aromatase inhibitors in cases of acute kidney injury, and further studies to explore the risk of AKI with aromatase inhibitors. AI’s should be used with caution in patient with pre-existing CKD, concurrent nephrotoxin use or a history of DI AIN, and monitoring of renal function should be undertaken when patients are commenced letrozole.
Abbreviations
AIN: Acute Interstitial Nephritis
AI: Aromatase Inhibitor
AKI: Acute Kidney Injury
DI: Drug Induced
Ethical Approval and Consent to Participate
This is covered under The Canberra hospital's human ethics committee. Patient consent was obtained prior to publication however all information is de-identified. Patient consented to participate.
Consent for Publication
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Availability of Data and Material
Not applicable.
Competing Interests
None.
Funding
None.
Author Contributions
All authors have made significant contributions to the generation of this submission and are in agreement with the contents of the manuscript. PP: Drafting manuscript and literature search and patient case history. ZW: Drafting manuscript and patient case history. MF: Histological data analysis of biopsy. SJ: Drafting and editing of manuscript.
Acknowledgements
Not applicable.
Article Info
Article Type
Case ReportPublication history
Received: Wed 29, Apr 2020Accepted: Wed 13, May 2020
Published: Mon 18, May 2020
Copyright
© 2023 Prianka Puri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.01.06
Author Info
M Fadia Prianka Puri Simon Jiang Wajih Z
Corresponding Author
Prianka PuriDepartment of Renal Medicine, The Canberra Hospital, Garran, Australia
Figures & Tables
Table 1: Renal function over time for patient.
|
|
Baseline 19/04/2018 |
On presentation 13/05/2018 |
Post treatment 22/06/18 |
|
Serum Creatinine |
68 µmol/L |
1069 µmol/L |
88 µmol/L |
Table 2: Urine assessment on presentation.
|
ANCA |
Not Detected |
|
Anti-nuclear antibodies |
Not Detected |
|
ENA |
Negative |
|
dsDNA |
Negative |
|
Rheumatoid factor |
Negative |
|
Anti CCP antibodies |
Negative |
|
Free kappa to lambda light chains ratio |
Normal |
|
C3 |
Normal |
|
C4 |
Normal |
Table 3: Autoimmune screen at presentation.
|
Protein concentration |
0.85 (ref <0.14) |
|
Creatinine |
4.0 |
|
Protein to creatinine ratio |
212 |
|
Leucocytes |
>100 x 106/L |
|
Erythrocytes |
>100 x 106/L |
|
Squamous epithelial cells |
<10 x 106/L |
|
Nitrite |
Negative |
|
Casts |
Nil |
|
Red cell morphology |
Normal |
|
Electrophoresis |
Mixed glomerulotubular protein |
|
Immunofixation |
No monoclonal immunoglobulin protein detected |

References
- Krishnan N, Perazella MA (2015) Drug-induced acute interstitial nephritis: pathology, pathogenesis, and treatment. Iran J Kidney Dis 9: 3-13. [Crossref]
- Baker RJ, Pusey CD (2004) The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 19: 8-11. [Crossref]
- Kalender ME, Sevinc A, Camci C, Turk HM, Karakok M et al. (2007) Anastrozole-associated sclerosing glomerulonephritis in a patient with breast cancer. Oncology 73: 415-418. [Crossref]
- Schanz M, Schricker S, Pfister F, Alscher MD, Kimmel M (2018) Renal complications of cancer therapies. Drugs Today (Barc) 54: 561-575. [Crossref]
- Troxell ML, Higgins JP, Kambham N (2016) Antineoplastic Treatment and Renal Injury: An Update on Renal Pathology Due to Cytotoxic and Targeted Therapies. Adv Anat Pathol 23: 310-329. [Crossref]
- Prendecki M, Tanna A, Salama AD, Tam FW, Cairns T et al. (2017) Long-term outcomein biopsy-proven acute interstitial nephritis treated with steroids. Clin Kidney J 10: 233-239. [Crossref]
- Moledina DG, Perazella MA (2017) Drug-Induced Acute Interstitial Nephritis. Clin J Am Soc Nephro 12: 2046- 2049. [Crossref]
- González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P et al. (2008) Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 73: 940-946. [Crossref]
